THE STUDY OF MULTI-COMPONENT CATALYSTS BASED ON OXIDES OF TRANSITION METALS DEPOSITED ON THE POLYMER MATRIX, IN THE OXIDATION OF SULFUR COMPOUNDS
Akhmadullin R.M., Biu Dinh Nhi, Akhmadullina A.G.
Abstract
The catalytic activity of the oxides of transition metals deposited on the polymer matrix in the oxidation of sodium sulfide was investigated. The synergistic effect of multicomponent catalysts based on oxides of transition metals in the oxidation of sodium sulfide was studied. It has been revealed that the mixed catalysts of ternary components have the highest activity in the oxidation of sodium sulfide, sodium hydrosulfide and ammonium sulfide.
Introduction
Environmental pollution is considered as the global warning and it needs the serious perception from entire countries on the world. Ecological norms are getting stricter and they impose the increased requirements to all of the processes that are performed at gas and oil refining plants and enterprises which are related to the production and processing of sulfur containing substances. [1].
The high contents of sulfide and sulfur in the sour alkaline solutions (SAS) do not allow them to biological treatment together with other wastewater. The most effective method of waste disposal is their local catalytic oxidation by air to less harmful compounds, such as thiosulfate, sulfate and thiosulfonate.
The catalytic activity in this case is present in the salts of metals with variable valence, such as Ni, Mn, Cu, Co, Fe… [2].
During the work we have studied oxidizing and catalytic characteristics of the oxides of the metals with variable valence applied to the polymer matrix. The polymeric heterogeneous catalyst has a high level of chemical stability, mechanical strength, and stable catalytic activity.
The catalytic activity of transition metals oxides in the oxidation of sodium hydrosulfide [3] and of mixed compositions of binary transition metal oxides deposited on the polymer matrix in the oxidation of sodium sulfide [4] were identified. To increase the activity of heterogeneous catalysts in the local catalytic SAS treatment, the catalytic activity of multi-component systems based on oxides of transition metals was investigated.
Experiments
Heterogeneous catalysts were synthesized by introducing transition metal oxides into the polymer matrix [5]. The polymeric heterogeneous catalysts contain catalytic component, its concentration is showed by the number (Example CuO- 20 – the polymeric heterogeneous catalyst contains 20 % Cuprum Oxide and 80 % polyethylene by mass).
The polymer used was a type of high polymer made from polyethylene, known commercially as KAZPELEN № 15313-003. The heterogeneous catalytic agent was represented by cubes with the following dimensions: 2х2х2 mm.
During experiments, the following oxides of the metals with variable valence were used:
- Manganese (IV) oxide (clean) under GOST 4470-79, measure. 1-2.
- Copper (II) oxide (clean for analysis) under GOST 16539-79.
- Nickel (II) oxide (clean) under GOST 4331-78.
- Chrome (III) oxide (clean) under GOST 2912-79.
- Cobalt oxide (II, III) (clean) under GOST 4467-79.
The sodium hydrosulfide and ammonium sulfide were prepared by dissolving in water, and the sodium sulfide was prepared by dilution of the 9-watered sodium sulfide in water under GOST 2053-77.
The oxidation of Na2S in an aqueous NaOH solution was made in an air bubbling vessel of oxidation. The oxygen was fed into the reaction solution with exact concentration of Na2S in presence of the catalytic agent which was being tested at a speed of 30,0 liter per hour-1. The solution inside the reactor was stirred at a speed of 1400 revolutions per minute-1. The temperature of the reaction solution was maintained at 60 °C with thermally controlled magnetic mixer.
The initial concentration of hydrosulfide, ammonium sulfide and sulfur in solution was 0.4% by weight. The concentration of the sulfur compounds in the solution was measured by potentiometric titration under GOST 22985-90 [6].
Results and Discussion
The catalytic activity of single-component of transition metal oxides in the oxidation of sodium sulfide [3] and of binary mixed compositions [4] is shown in accordance with the ranges:
MnO2>CuO> Co3O4> NiO >Cr2O3 (1)
MnO2/CuO > CuO/ Co3O4 > Co3O4/ NiO > NiO/ Cr2O3 (2)
In this case, a higher activity of the binary mixed compositions was explained by electron transfer between cations of single-phase oxides of transition metals [7, 8]. Therefore, in order to increase the activity of the catalysts was studied by us in addition. The activity of heterogeneous catalysts consisting of mixtures of binary compositions of transition metal oxides deposited on the polymer matrix was studied in order to increase the catalytic activity (Table 1).
The mixture containing the binary compositions of transition metal oxides with the highest individual activity shows the maximum catalytic activity in the oxidation of sodium sulfide. It is shown that the combined operation of binary compositions increases the synergy effect. The increasing activity of mixed catalysts associated with the number of binary compositions, i.e. the mixture of the four catalysts shows high rate in the oxidation of sodium sulfide (see column 6).
Recently, so much attention is given to multi-component oxide catalysts which have high activity and selectivity in the oxidation of hydrocarbons, depending on the type and number of oxides [9]. Таb. 1 The catalytic activity of mixtures of binary compositions of transition metal oxides in the oxidation of Na2S.
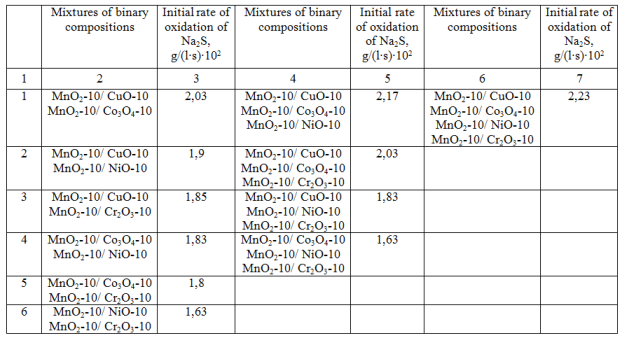
The catalytic activity of multi-component catalysts of transition metal oxides in the oxidation of sodium sulfide is presented in Table. 2. The highest rate of oxidation of sodium sulfide is observed in the presence of catalysts which contain three oxide components in the polymer matrix. The further increasing the number of oxides in the catalysts to 4 and 5 decreases the initial oxidation rate, which is probably explained by lower concentrations of transition metal oxides on the polymer surface of the heterogeneous catalysts. It is observed experimentally that the minimum required concentration of the metal oxide was 5.0% by mass in the polymer matrix.
Таb. 2 The activity of multi-component catalysts of transition metal oxides in the oxidation of Na2S.
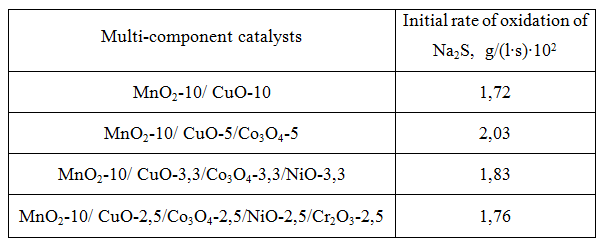
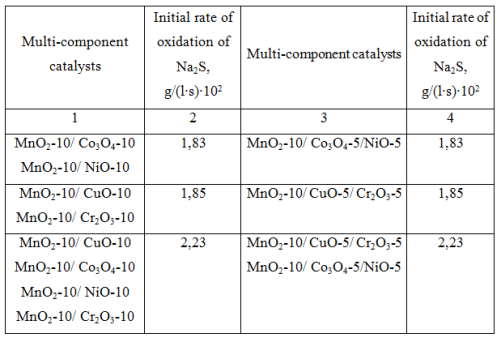
Then the activity of heterogeneous catalysts consisting of mixtures of ternary compositions of transition metal oxides deposited on the polymer matrix was investigated (see Table 3). These results indicate the possibility of replacing the double mixed binary compositions (table 3- column 1) with individual triple catalyst composition (column 3).
Таb. 3 The activity of multi-component catalysts of transition metal oxides in the oxidation of Na2S.
It is known [10] that hydrosulfide and sodium sulfide are oxidized slowly in the absence of catalysts. Ammonium sulfide is not very stable, rapidly is oxidized even under normal conditions.
The investigation results of the oxidation of sodium sulfide, hydrosulfide and ammonium sulfide in the presence of heterogeneous oxide catalyst and without catalyst are shown in Fig. 1
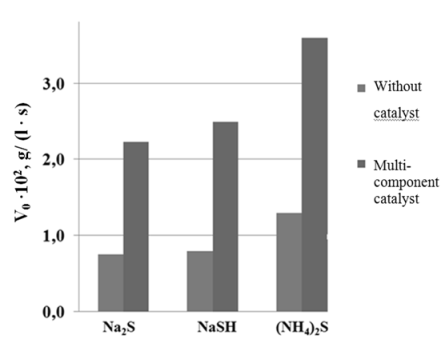
Fig. 1 Influence of heterogeneous oxide catalyst on initial oxidation rate (V0)- sodium sulfide, hydrosulfide and ammonium sulfide.
The investigation results show that the catalyst accelerates the oxidation rate of sulfide, sodium hydrosulfide and ammonium sulfide. Thus, the rate of oxidation of sodium hydrosulfide and ammonium sulfide in the presence of the catalyst is 2.9 times higher than without catalyst, and catalytic oxidation rate is 2.7 times higher than the rate of non-catalytic oxidation in the case of sodium sulfide.
Conclusion
1. The multicomponent catalysts of transition metal oxides and their mixtures deposited on the polymer matrix have the high catalytic activity in the oxidation of sodium sulfide.
2. The increasing the rate of oxidation of sodium sulfide by using mixture of bi- and tri- component oxide catalysts was determined.
3. The minimum required concentration of transition metal oxides in the polymer matrix is 5.0 % by weight.
4. It is shown that heterogeneous oxide catalysts have high activity in the oxidation of sulfur compounds, including sodium sulfide, hydrosulfide and ammonium sulfide. The highest activity of the catalyst is shown in the oxidation of sodium hydrosulfide and ammonium sulfide.
REFERENCES
[1] A.G. Akhmadullina, A.M. Mazgarov, E.G. Ioakimis, I.J. Mongait. Wastewater treatment refineries. – M.: Chemistry, 1985. – p. 1-35.
[2] M. R. Hoffinan, B.C. Lim. Kinetics and mechanism of oxidation jf sulfide by oxigen: Catalitics by homogenious metalphtalocyanyne complexes / / Environmental Science Technology. 1979. – V.13. – N. 11. – P. 1406-1414.
[3] R.M. Akhmadullin, Bui Dinh Nhi, A.G. Akhmadullina, Ya.D. Samuilov The catalytic activity of transition metals oxides deposited on the polymer matrix, in the oxidation of sodium hydrosulfide – Bulletin Kazan Technological University. V. 15, № 1, 2012, p. 50-54.
[4] R.M. Akhmadullin, Bui Dinh Nhi, A.G. Akhmadullina The study of binary mixed compositions of transition metal oxides deposited on the polymer matrix, in the oxidation of sodium sulfide – Butlerov Communications. T.30, № 5, 2012, p. 94-97.
[5] AS № 1041142, Bulletin number 34, 1983. The catalyst for the oxidation of sulfur compounds and its preparation / A.G. Akhmadullina, A.M.Mazgarov, M.I.Alyanov, V.V.Kalacheva, I.K.Hruscheva, G.M.Nurgalieva, G.A.Ostroumova, A.F.Vildanov.
[6] GOST 22985-90. Liquefied hydrocarbon gases. Method for determination of hydrogen sulfide and mercaptan sulfur
[7] S. Veprek, D.L. Cocke, S. Kehl and H.R. Oswald. J. Catal. 100, 1986, 250.
[8] F.C. Buciuman, F. Patcas, T. Hahn. A spillover approach to oxidation catalysis over copper and manganese mixed oxides. Chem. Eng. Proc. 38, 1999, P. 563-569.
[9] Patent US5470815 – Multicomponent oxide catalyst.
[10] I.M. Zamyshlyaeva. Purification of industrial waste water. Collection № 4., 1969, 248.