Abstract — The antioxidant performance of a sterically hindered bisphenol antioxidant, 4,4′-bis(2,6-di-tert-butylphenol), in model reactions of accelerated aging of carbon-chain polymers (polypropylene, isoprene rubber) was studied. This antioxidant was examined by differential scanning calorimetry and Wallace plasticity measurements in comparison with known commercial phenolic and amine stabilizers. High performance of 4,4′-bis(2,6-di-tertbutylphenol) as antioxidant was revealed.
DOI: 10.1134/S1070427215050171
ISSN 1070-4272, Russian Journal of Applied Chemistry, 2015, Vol. 88, No. 5, pp. 833−838. © Pleiades Publishing, Ltd., 2015. Original Russian Text © R.M. Akhmadullin, D.R. Gatiyatullin, L.A. Vasil’ev, A.G. Akhmadullina, N.A. Mukmeneva, E.N. Cherezova, Mingshu Yang, 2015, published in Zhurnal Prikladnoi Khimii, 2015, Vol. 88, No. 5, pp. 792−797.
The production and consumption of polymeric items steadily grows. Nevertheless, the demand for polymers is not always met by their supply, and therefore it becomes necessary to prolong the operation life of ready items. The most widely used way to prolong the operation life of polymeric items is introduction of stabilizers, antioxidants (AOs).
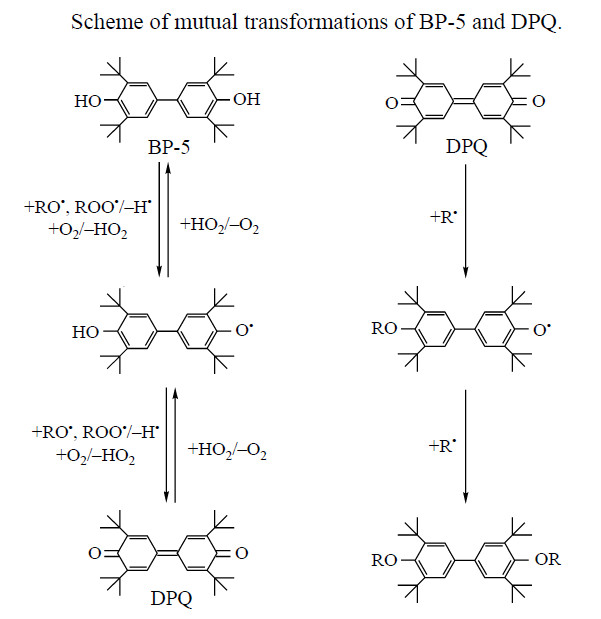
Phenolic antioxidants find increasing use in the practice of polymer stabilization. Among them, bisphenol (BP) antioxidants exhibiting high performance should be particularly mentioned [1–3]. 4,4′-Bis(2,6-di-tertbutylphenol) (BP-5), a BP AO, was synthesized previously by base oxidative dehydrogenation of hydroquinone with 3,3′,5,5′-tetra-tert-butyl-4,4′-diphenoquinone (DPQ). BP-5 is permitted as an additive to polymers for medical purpose [5–7]. This fact largely determines the interest in this stabilizer from the viewpoint of both the development of effi cient methods for its synthesis and the evaluation of its inhibiting power in aging of polymers of various structures [8]. A signifi cant feature of BP-5 is that, when inhibiting the oxidation of carbon-chain polymers, it transforms into 3,3′,5,5′-tetra-tert-butyl-4,4′-diphenoquinone, which can be reduced again to the initial BP-5 [8]. This cyclic process takes place until the stabilizer is completely exhausted. In the process, BP-5 reacts with peroxy radicals ROO• [9] (see scheme), and its oxidized form, DPQ, is a scavenger of alkyl radicals [8, 10]. It is highly probable that specifi cally these mutual transformations are responsible for the presence of compounds exhibiting synergistic AO effect [11] and ensuring high activity of BP-5 in inhibition of the thermal oxidation of polymers. This can be proved by the mechanisms of the inhibiting action of BP-5 and DPQ in free radical oxidation of carbon-chain polymers, given in [12]: reaction of alkyl radicals (R•) with quinones and methylenequinones
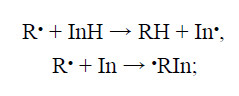
reaction of peroxy radicals (ROO•) with sterically hindered phenols
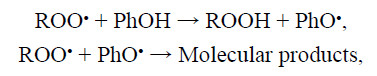
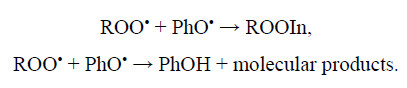
With the aim of characterizing BP-5 more completely and expanding the prospects for its use, we performed in this work a comparative study of the stabilizing performance of BP-5 in isoprene rubber and polypropylene. Synchronous evaluation of the stabilizer performance in a rubber and in a plastic is topical from the viewpoint of the development of thermoelastoplastic materials.
EXPERIMENTAL
Synthesis of 4,4′-bis(2,6-di-tert-butylphenol). An autoclave was charged with 50.0 mL of toluene, 0.0122 mol of DPQ, 0.0022–0.0122 mol of hydroquinone, and a 0.75 M aqueous NaOH solution. The mixture was heated to 160°С. The rotation rate of the magnetic stirrer was 1400 rpm. The reaction mixed decolorized in the course of the reaction.
After the reaction completion, the reactor was cooled to 70°С. The reaction mixture consisted of two layers: the upper toluene layer with the dissolved BP-5 and the lower aqueous alkaline solution. BP-5 precipitated from the toluene fraction at room temperature; it was fi ltered off and dried. A white powder was obtained, Тm = 184–185°С. The kinetic curves of the conversion of DPQ into BP-5 were plotted on the basis of the data on the light absorption of the reaction solutions of DPQ, obtained with an Ekros PE5300V spectrophotometer (λ = 540 nm). The component composition of the reaction mixture was analyzed with a Khromatek–Kristall 5000 gas chromatograph.
Study of 4,4′-bis(2,6-di-tert-butylphenol)properties. The AO performance of the stabilizer in SKI-3 synthetic rubber was evaluated by the plasticity retention index (PRI) at thermal aging (130°С, 40 min) in accordance with ISO 2930:2009 (Sterlitamak Petrochemical Plant). The stabilizer in the form of a toluene solution was introduced into SKI-3 polymerizate; the solution was degassed in an air drier at 75°С. The stabilizer concentration in the rubber was from 0.05 to 0.3 wt %. Samples of polypropylene (PP) were submitted by CIBA (China).
The AO performance of stabilizers in PP was examined in the CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences (Beijing). Polypropylene samples were prepared either by introducing AO into a PP melt (Sinopec Yanshan B1101, melt fl ow rate 0.5 g min–1) or by introducing it in a twoworm extruder (Haakepolylab OS Rheodrive 7, length/diameter 25, diameter 16 mm) at a worm rotation rate of 100 rpm. The working temperature of extruder zones was 180, 210, and 210°С. Antioxidants were introduced into PP in an amount of 0.3 wt %.
After cooling in water, the extrudates were granulated and dried. The induction periods of oxidation (IPO) were determined by differential scanning calorimetry (DSC) using Perkin–Elmer DSC 7 software. The induction period of oxidation of PP samples was determined in accordance with ISO 11357-1:2009. Polypropylene samples were kept for 5 min at 60°С in a nitrogen stream (50 mL min–1). Then, the temperature was raised from 60 to 200°С at a rate of 20 deg min–1, and the samples were again kept in a nitrogen stream at the same fl ow rate, 50 mL min–1. After that, nitrogen was replaced by oxygen (fl ow rate 50 mL min–1). The sample oxidation was detected as a sharp increase in the thermal effect caused by the exothermic oxidation reaction. The time between the start of feeding oxygen and the start of heat evolution due to the exothermic process was considered as IPO of PP.
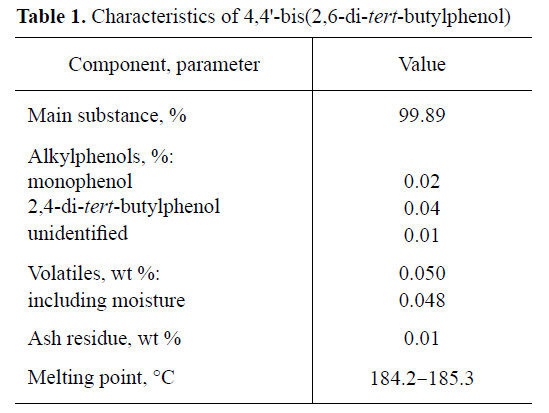
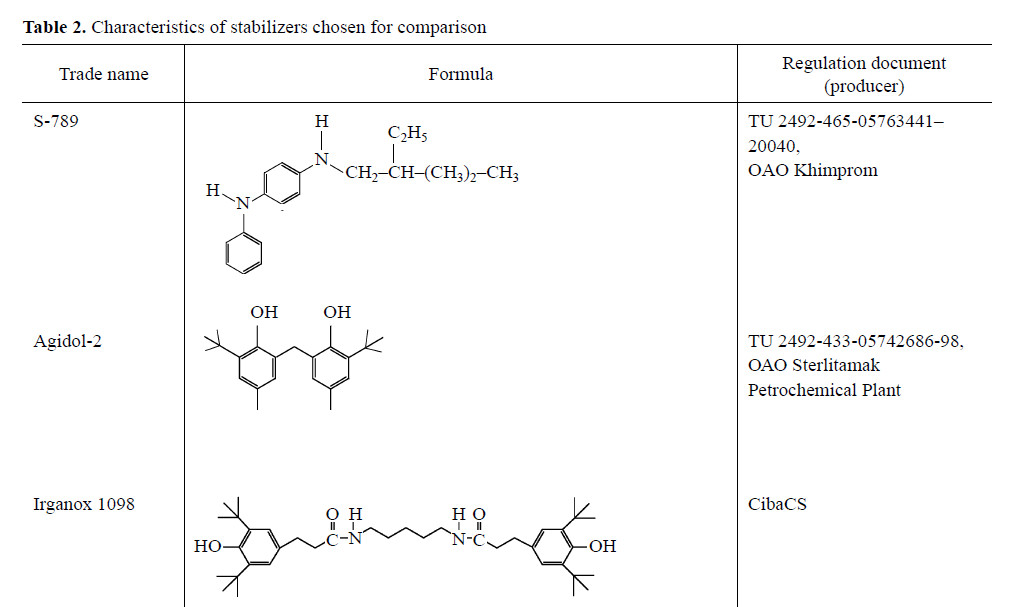
The characteristics of BP-5 are given in Table 1. The stabilizers considered for comparison are presented in Table 2.
RESULTS AND DISCUSSION
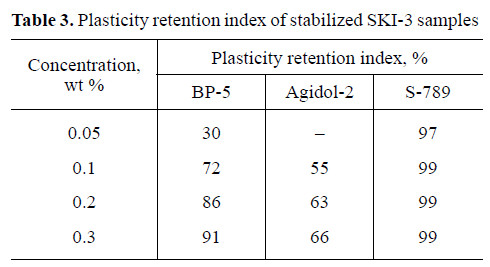
Testing of AO under the conditions of SKI-3 aging showed that, at concentrations of 0.1–0.3 wt %, BP-5 ensures higher plasticity retention index for rubber than does Agidol-2. On the other hand, at low temperatures BP-5 is less effective than S-789 amine antioxidant (Table 3).
The induction periods of PP oxidation in the presence of BP-5 and of commercial AOs are presented in Fig. 1.
The data obtained show that the stabilizing performance of BP-5 is 3 times higher than that of Irganox 1098 (Figs. 1–3, curves 3). In Irganox 1098, two 2,4-di-tertbutylphenyl groups are linked by a linear chain in which two amide residues are remote from each other. This structure makes Irganox 1098 incapable of reversible phenol–quinone redox transformations. In addition, bulkier Irganox 1098 is less mobile; hence, the probability of collisions of phenol hydroxy groups with the oxidizing agent decreases. Apparently, bulky tetramer Irganox 1010 (Figs. 1–3, curves 5) is 3 times less effi cient as AO than BP-5 for the same reasons.
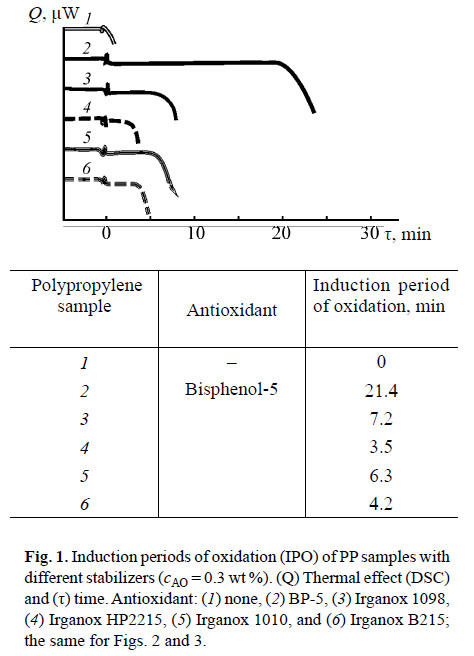
Mixed AO Irganox HP2215 (Figs. 1–3, curves 4) exhibits 6 times lower performance than BP-5 does. It is known that different phenolic antioxidants compete with each other under the conditions of oxidant defi ciency [13]. Most probably, the action of polyphenols in Irganox HP2215 may be determined by competing inhibition. The relative enhancement of the performance of binary AO Irganox B215 (Figs. 1–3, curves 6), which consists of Irganox 1010 and Irgafos 168 in 1 : 2 ratio, indirectly confi rms the above hypothesis. On the other hand, the AO performance of Irganox B215 is 5 times lower than that of BP-5.
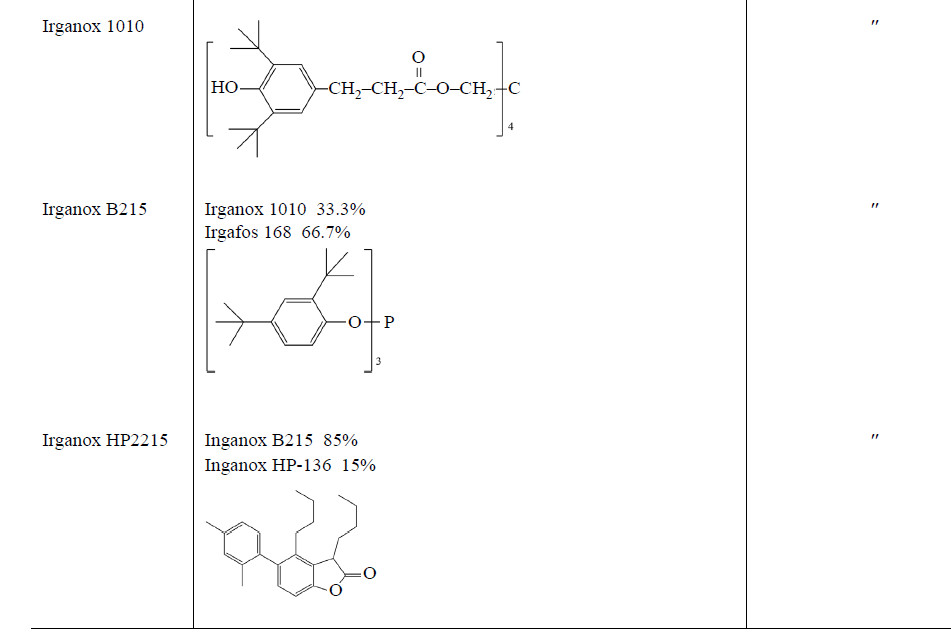
Apparently, the decisive factor of high AO performance of BP-5 is in situ formation of the phenol–quinone synergistic mixture.
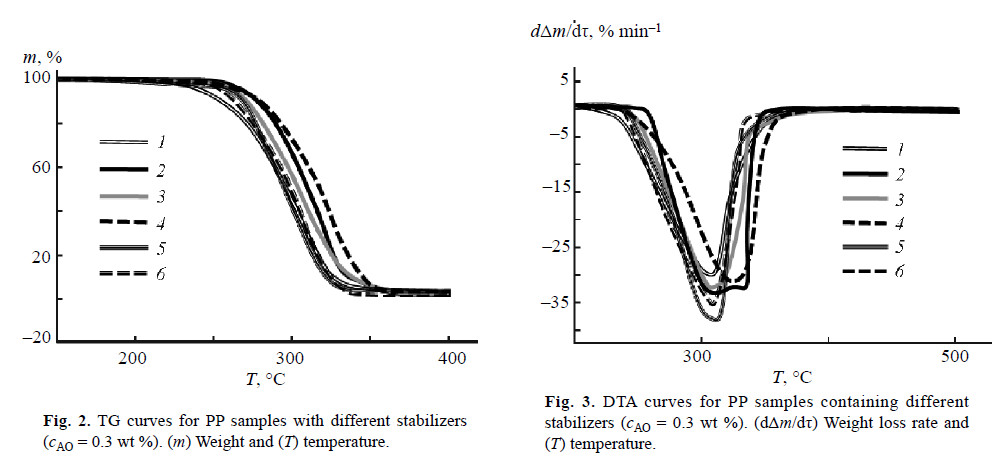
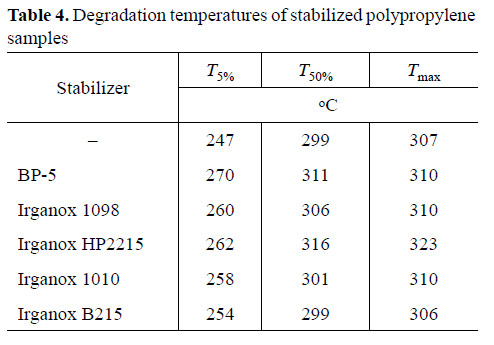
It follows from the results of thermogravimetric and differential thermal analysis (Figs. 2, 3) that introduction of BP-5 into PP increases the temperatures of its degradation and weight loss onset (Table 4). This means that the operation temperature interval of the polymer is expanded and its operation life is made longer.
On the whole, BP-5 is a promising effective antioxidant for a wide range of polymers and their blends.
CONCLUSIONS
(1) BP-5 ensures higher plasticity retention index of SKI-3 synthetic rubber than does Agidol-2 phenolic antioxidant and is comparable in performance with S-789 amine antioxidant.
(2) In polypropylene stabilization, BP-5 considerably surpasses in performance other antioxidants used in practice, as judged from the induction period of oxidation. Thus, BP-5 can be recommended as additive for production of polypropylene items for various purposes and of thermoelastoplastics.
REFERENCES
- Bashkatova, T.V., Miryasova, F.K., Cherezova, E.N., and Bukharov, S.V., Russ. J. Appl. Chem., 2005, vol. 78, no. 7, pp. 1110–1114.
- Saigitbatalova, S.Sh., Cherezova, E.N., Balabanova, F.B., et al., Butlerovsk. Soobshch., 2013, vol. 36, no. 10, pp. 60–64.
- Roginskii, V.A., Fenol’nye antioksidanty. Reaktsionnaya sposobnost’ i effektivnost’ (Phenolic Antioxidants. Reactivity and Performance), Moscow: Nauka, 1988.
- Akhmadullin, R., Gatiyatullin, D., Akhmadullina, A., et al., Res. J. Pharm., Biol. Chem. Sci., 2014, vol. 5, no. 6, pp. 494–502.
- Wattenberg, L.W., Coccia, J.B., and Lam, L.K.T., Cancer Res., 1980, vol. 40, no. 8, part 1, pp. 2820–2823.
- Duong, H.T.T., Antao, S., Ellis, N.A., et al., Brain Res., 2008, vol. 1219, pp. 8–18.
- Kim, H.B., Shanu, A., Wood, S., et al., Free Radical Res., 2011, vol. 45, no. 9, pp. 1000–1012.
- Akhmadullin, R.M., Nugumanova, G.N., Mukmeneva, N.A., et al., Kauch. Rezina, 2006, no. 10, pp. 12–14.
- Shanina, E.L., Zaikov, G.E., and Mukmeneva, N.A., J. Appl. Polym. Sci., 2003, vol. 87, no. 14, pp. 2226–2229.
- Görner, H., J. Photochem. Photobiol., 2010, vol. 86, no. 6, pp. 1202–1207.
- Emanuel’, N.M. and Buchachenko, A.L., Khimicheskaya fizika molekulyarnogo razrusheniya i stabilizatsii polimerov (Chemical Physics of Molecular Degradation and Stabilization of Polymers), Moscow: Nauka, 1988.
- Denisov, E.T. and Denisova, T.G., in Handbook of Antioxidants, New York: CRC, 2000, pp. 1–289.
- Vershinin, I.V., Vlasova, I.V., Tsypko, T.G., et al., J. Anal. Chem., 2011, vol. 66, no. 7, pp. 595–602.