HETEROGENEOUS CATALYTIC DEMERCAPTIZATION OF LIGHT HYDROCARBON FEEDSTOCK
A. G. Akhmadullina, B. V. Kizhaev, G. M. Nurgalieva, I. K. Khrushcheva and A. S. Shabaeva
UDC 665.664.22:541.128.13
The Demer-Luvs process has been put into commercial operation in the KT-1 unit at the Mazheikyai refinery for the demercaptization of a butane-butene fraction (BBF). In this process, in contrast to the Merox process [1] and VNIIUS-12 process [2], the mercaptide-containing caustic solution is not regenerated with a homogeneous phthalocyanine catalyst, but rather by the heterogeneous phthalocyanine catalyst KS-2B, which has a polymeric matrix as a support [3]. The use of this heterogeneous catalyst eliminates the operation of preparing a catalyst complex; the service life of the phthalocyanine catalyst has been increased from 3-4 months to 3-5 years; and a further benefit is the elimination of any discharge of cobalt phthalocyanines along with the spent caustic into the refinery wastewater.
The KS-2B catalyst is prepared in accordance with Specification TU 383047-91 in a form that is convenient for operation, namely, packing elements with a well-developed geometric surface, such as Pall rings, lobes, etc. The packing is charged to the regenerator as a single layer of dumped packing. In addition to its function as a catalyst, it serves as an effective packing that improves the mass transfer between the oxidizing gas and the caustic solution that is being regenerated. The use of the polymeric support gives the catalyst certain advantages in service, such as resistance to alkaline hydrolysis, high mechanical strength, and low density; also, the hydrodynamic conditions in the reactor are favorable with this type of catalyst.
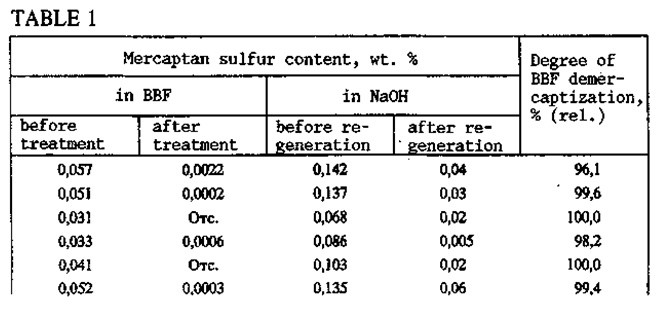
With the KS-2B catalyst, the BBF is thoroughly demercaptized (Table 1). The mercaptide-containing solution is regenerated under mild conditions: temperature 35-40°C, gauge pressure 0.12-0.15 MPa, air consumption 100-180 m3. Six months of operation of the KT-1 unit have demonstrated the high efficiency and reliability of the Demer-Luvs process. During that period and up to the present time, treatment of BBF with a mercaptan sulfur content of 0.08-0.019% has regularly given a product with 0.0005-0.001% mercaptan sulfur by weight.
The service life of the caustic solution, i.e., the length of the operating period before replacement with fresh solution becomes necessary, has been extended to 6-7 months, indicating that there is very little formation of acidic products in the process of caustic regeneration on the KS-2B catalyst. This result confirms laboratory data that had been obtained previously on the composition of the products from catalytic oxidation of sodium mercaptides on homogeneous and heterogeneous phthalocyanine catalysts [4]. According to those data, the yields of products of the type of RSO„Na (where n = 1-3) from the oxidation of sodium salts of ethyl, propyl, and butyl mercaptans on the catalysts KS-1 and cobalt disulfophthalocyanine in the VNIIUS-12 process at the Salavat and Novokuibyshevsk Petrochemical Combines were approximately identical: 5-10% of the original mercaptan sulfur content.
The same sort of product is formed when using a new catalyst, cobalt dichlorodihydroxyphthalocyanine, known as IVKAZ, which is being used for BBF demercaptization at the Ryazan’ refinery [5]. Apparently, a phenomenon that was noted in [4] occurs in the course of regenerating the caustic solution: Salts of sulfuric acids that are formed in small quantities are transformed to water-soluble, neutral, oxygen-containing products; and this prevents any rapid consumption of active caustic in the system. This is probably the reason for the extended service life of the caustic solution in the Demer-Luvs process, in spite of the rather low yield of disulfides in oxidation of mercaptides on the polymeric phthalocyanine catalyst KS [4].
The polar nature of the oxygen-containing products that are formed evidently facilitates the extraction of mercaptans from the BBF by the caustic solution; and as a consequence, the extracting capability of this solution for mercaptans is maintained even when the concentration of active caustic drops to 4% by weight or lower. An analysis of caustic solution that had been in use in the demercaptization system for more than two months indicated that it did not contain any significant quantities of sulfinic or sulfonic acids.
Because of the use of the phthalocyanine catalyst anchored on the polymeric support in the regenerator, contact between the treated product and cobalt phthalocyanine in the extractor is eliminated, so that there is no oxidation of mercaptide sulfur in the extractor or contamination of the treated product with disulfides. Entry of disulfides into light hydrocarbon feedstocks is undesirable, especially in products in which die presence of a liquid residue is not permissible, and in which the total sulfur content is strictly limited.
Disulfides make it into the treated product as a result of carryover from the regenerator as a consequence of poor washing and separation from the caustic, and also as a result of direct formation in the extractor by oxidation of mercaptans, either by cobalt phthalocyanine or by oxygen that enters with the caustic from the regenerator, either chemisorbed on the catalyst or in the dissolved state.
This is possible if the regeneration of the mercaptide-containing caustic is too thorough, which is difficult to avoid when using the highly active homogeneous phthalocyanine catalyst. Consideration must also be given to the considerable variations of the initial concentration of mercaptan sulfur in the products being treated, and to the difficulty of any close control of the temperature and the air input rate in the regenerator.
Thus, for the demercaptization of a light hydrocarbon feedstock, if the total sulfur content and the liquid residue in the product are strictly limited, and also if there is no need for the production of disulfides, the Demer-Luvs process is preferably performed with a heterogeneous phthalocyanine catalyst of the KS-2B type. This process has been introduced in the Moscow and Novo-Baku refineries for demercaptization of BBF from units for exhaustive processing of crude oil, units of the G-43-107 type.
REFERENCES
1. U.S. Pat 4,498,978.
2. USSR Inventor’s Certificate 823,418.
3. USSR Inventor’s Certificate 1,041,142.
4. A. G. Akhmadullina, L. N. Orlova, I. K. Khrushcheva, et al., Zh. Prikl. Khim., No. 1, 53-57 (1989).
5. A. F. Vil’danov, S. A. Gorokhova, A. M. Mazgarov, et al., Neftepererab. Neftekhim., No. 8, 15-16 (1991).