Introduction
For the past decades, finding suitable and effective methods to reduce air and water pollution has been considered as one the most important and urgent tasks. In the industry, the petrochemical sector is one of the leading contributors to the world economy, but also one of the main sources of air and water pollution [1-2]. Recent reports about strategic development of heavy industries have placed the petrochemical sector at the second place in the volume of wastewater discharging into the environment as well as in the use of water resources from all industry sectors [1-2].
Industrial wastes from petrochemical activities defined by the presence of sulfur-containing compounds such as inorganic sulfides and mercaptides are called sulfur-alkaline waste (SAW). Due to the high toxicity, SAW is not allowed to be released into the environment, not even after being diluted or treated by other industrial effluents. Thus, a separate system for collecting and purifying SAW is required for petrochemical activities [3].
SAW can be treated by different methods, such as neutralization, oxidation methods, biological methods, etc. In reality, in most of oil refineries, high pH SAW is directly transferred to biochemical treatment plants after discharged into the sulfur-alkaline sewage system (without local purification facilities). Because the optimal pH range for the biological system is between 7 and 7.8, the wastewater is usually neutralized with sulfuric or hydrochloric acid. This leads to forming and releasing free hydrogen sulfide, which is then combusted to SO2 and discharged into the atmosphere, causing a serious environmental problem [4].
In order to reduce the amounts of sulfur compounds and spent-alkaline in SAW, the carbonization method is periodically used. The main objective of this method is to neutralize and acidify the wastewater using concentrated carbon dioxide with the simultaneous production of a soda as well as releasing acid compounds into the free state.
Na2S + CO2 + H2O = Na2CO3 + H2S (1)
2NaHS + CO2 + H2O = Na2CO3 + 2H2S (2)
Similar to the neutralization method, the environmental pollution caused by the combustion of free hydrogen sulfide is serious disadvantage to be considered.
In the secondary petroleum refining processes, aqueous condensate from hydrogen sulfides and hydrosulfides the oil refineries are usually treated using a stripping method, in which the wastewater is heated to its boiling point at operating pressure in a column apparatus with the use of valve plates [6]. During this treatment, dissolved hydrogen sulfide will be collected by either a dry gas or a propane stream. Disadvantages of the stripping method are high-energy intensity and difficulty to recycle the formed gas containing hydrogen sulfide.
Chemical oxidation methods, in which inorganic sulfides are oxidized by strong oxidizing agents (for example KMnO4, K2Cr2O7, O3, Ca(Cl)2O2, H2O2 and Cl2) to form less toxic oxygen-containing products such as thiosulfate, sulfite and sulfate, have been widely used in petrochemical industry to purify SAW from the sulfides sulfur, [7, 8]. However, using these oxidants leads to an increase in the treatment cost and the formation of additional pollutants as well as equipment corrosion. Both from an economic and ecological point of view, the oxygen (air) is the most reasonable oxidant for sulfide degradation. Nevertheless, oxygen has a low purification efficiency in treating SAW, which can be greatly enhanced by adding catalysts.
Applying catalyst to the oxidative degradation process of SAW remarkably reduces energy intensity of treatment process as well as increases its efficiency [9-11]. Transition metals and their compounds are widely considered as the potential catalysts for selective oxidation of sulfide in petrochemical industry [12-14]. Similarly, the transition-metal phthalocyanines (primarily cobalt phathalocyanines and their derivatives) are one of the most common catalysts used in sulfide sulfur oxidation processes. Non-metals, transition-metal oxides (such as SiO2, Al2O3, Fe2O3, MnO2, TiO2) and their derivatives such as mullit – CuMgCr2O4, ferrite – MFe2O4 (M = Fe, Cu and Co) are often used as heterogeneous catalysts in liquid-phase sulfide oxidation [12 -16]. However, using these catalysts also has several serious disadvantages such as high cost of catalyst components and relatively low catalytic activity in treating concentrated sulfide wastewater, and most of all the possible pollution possibly caused by heavy metals dissolved in treated wastewater.
For the past few years, quinones and their derivatives, which has been widely applied to sulfide purification, particularly in Japan and the UK, have become an interesting target in the search for new catalysts for sulfide treatment [17-18]. In 1979, Ueno H [18] have reported that hydroquinone, 1,2-naphthoquinone-4-sulfonic acid sodium salt, 1,4-naphthoquinone and their mixtures are capable of drastically enhancing the sulfide oxidation. The disadvantages of using these catalysts are high catalyst losses, labor-intensity of catalyst separation and hard to recover after effluent treatment [19]. Iwasawa in 1977 has showed that the polynaphthoquinone also has a high catalytic efficiency for sulfide oxidation [20]. However, during the reaction, disposition of formed sulfur on the surface of the polynaphthoquinone-catalyst was found, which possibly lead to a decrease in its activity.
In order to eliminate those mentioned above disadvantages, we propose a new quinone-catalyst based on 3,3′,5,5′-tetra-tert-butyl-4,4′- stilbenequinone dissolved in kerosene fraction (hereafter, referred to as stilbenequinone) that has a high catalytic activity, high selectivity and stability in alkaline media. The choice of kerosene fraction as the catalyst carrier is due to its low aqueous solubility as well as low volatility. Moreover, the good solubility of stilbenquione in kerosene fraction is also considered as an advantage. The aim of this study is to characterize kinetics, mechanism and regulation of the catalytic cycle of liquid-phase sulfide oxidation in the presence of a new catalyst based on stilbenequinone and to evaluate the factors affecting the reaction rate.
Experimental Section
Catalyst Preparation
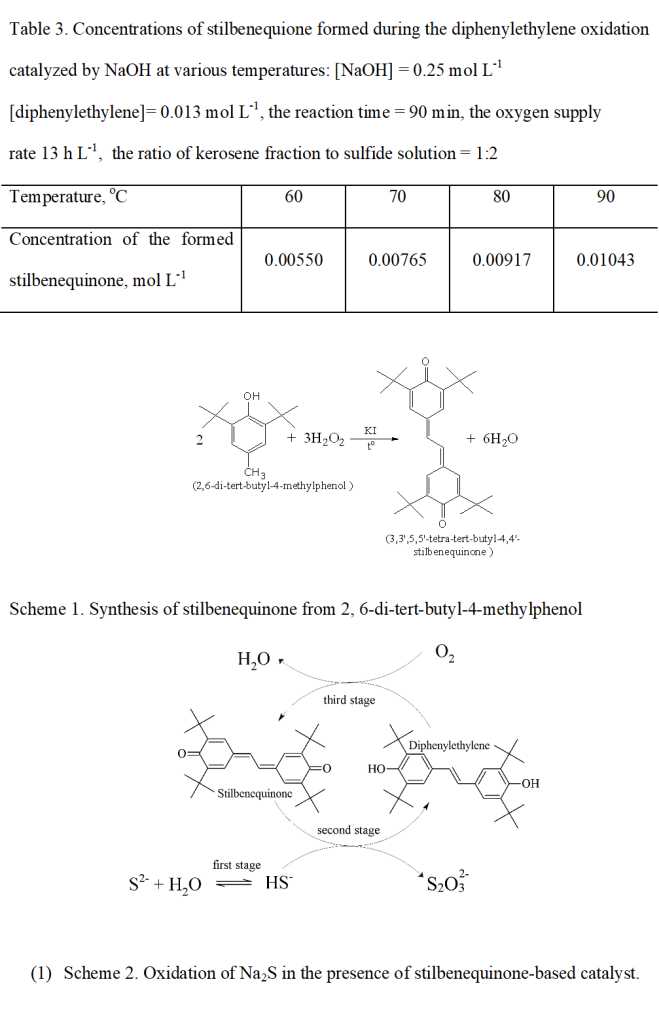
The catalytic component – 3,3′,5,5′-tetra-tert-butyl-4,4′-stilbenequinone was synthesized by oxidation of 2,6-di-tert-butyl-4-methylphenol with hydrogen peroxide in the presence of a catalyst based on potassium iodide (Scheme 1) as described in [21].
(Scheme 1)
Analysis results: melting point 315 °C, IR spectrum, ν, cm -1: 3003 (CH (Ar)), 1605 2952 2909 2865 (Me)), 1640 1605 ((Ar) = C-C = (Ar)), 1605 ( C = O,), 1600 1454 (C = C (Ar)), 1360 1256 t-Bu; 1H NMR (CD3) 2SO, 600 MHz), δ ppm: 1.42 (s, 36H, C (CH3)); 6.54 (s, 2H, = CH); 7.19 (s, 4H, C6H2). Found C, 82.80, 82.56; H, 9.73; O, 7.47, 7.71 C30H42O2; Calculated: C, 82.55; H, 10.15; O, 7.3.
Reagents
During the experiments, the following reagents were used: technical gaseous oxygen in cylinders (Russia, GOST 5583-78), technical gaseous argon in cylinders (Russia, GOST 10157-79), kerosene fraction (Russia, GOST 10227-2013). Aqueous sulfide solutions were prepared by dissolving amounts of reagent grade Na2S·9H20 (Russia, GOST 2063-77) in deoxygenated distilled water. A solution of sodium hydrosulfide was prepared according to the method [22].
Catalytic oxidation of sulfide sulfur
Oxidation of inorganic sulfides was performed in a 150 mL three-necked cylindrical glass reactor. Sixty milliliter of reaction mixture contained inorganic sulfide solution with concentration of 0.143 – 1.750 mol L-1 and kerosene fraction was loaded into the reactor in the presence of certain amount of the catalyst component. The ratio of kerosene fraction to sulfide solution was changed from 1:40 to 1:1. Oxygen from the cylinder was injected into the reaction solution at 0-13 L h -1. The reaction temperature was maintained in the range of 50-90 °C and the reaction solution was stirred at speed of 0-1400 rpm by a thermally controlled magnetic stirrer.
Experimental-analytical methods
The quantitative content of inorganic sulfide was determined by potentiometric titration method UOP-209-00 (USA). The concentrations of thiosulfate and sulfite, as well as of hydrogen peroxide were identified by the idometry method proposed in [23, 24]. The concentration of sodium sulfate was determined using spectrophotometry (Eros PE5300B, regime A, wavelength l=450 nm path length L=50.0 mm). Similarly, the stilbenequinone concentration was also determined by this method ((Eros PE5300B, regime A, wavelength l=500nm, path length L=10.0mm). Infrared spectra (IR) of substances were recorded using Perkin Elmer Spectrum Two FTIR spectrometer, whereas 1H Stilbenequinone NMR spectra were recorded using Bruker Avance 600 spectrometers at an operating frequency of 600 MHz. The melting point of the substances was determined by Buchi M-560 instrument. Elemental analysis of stilbenequinone was performed using workstation Auriga Cross Beam.
Results and Discussion
Kinetics of liquid-phase oxidation of Na2S in the presence of stilbenequinone based catalyst
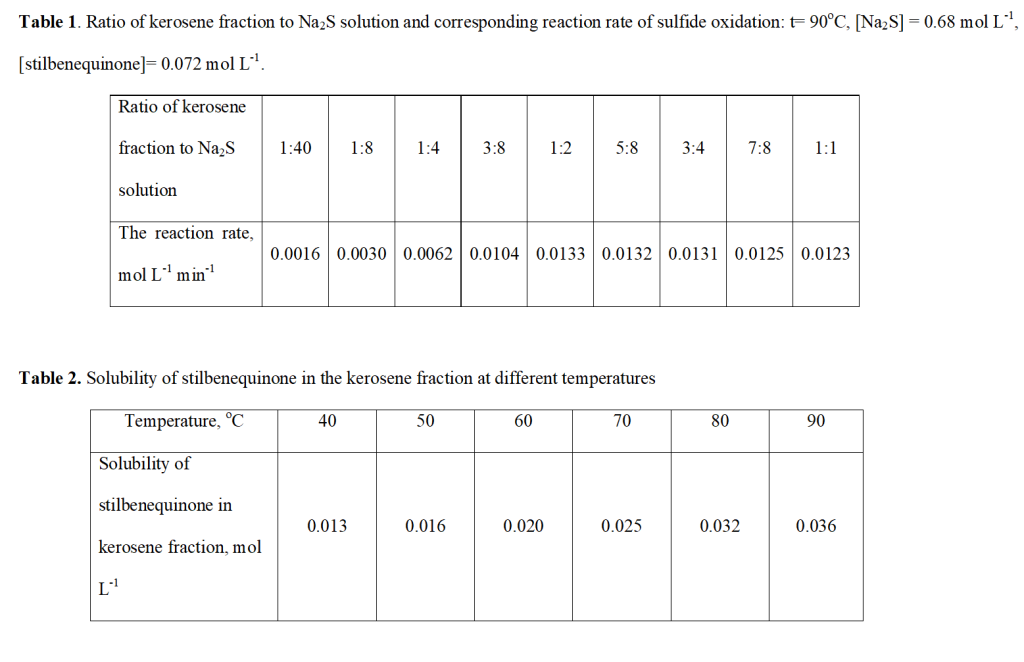
The reaction of liquid-phase sulfide oxidation in the presence of a stilbenequinone-based catalyst takes place in a three-phase system “oxygen – hydrocarbon fraction – aqueous alkaline solution of inorganic sulfide”. Therefore, the catalytic oxidation rate of sulfide sulfur depends not only on the chemical reaction rate, but also on the reactant diffusion rate. The process of controlling catalytic sulfide oxidation in the presence of stilbenequione was determined in a sequence of experiments, in which the initial volumetric concentration of oxygen diluted with argon and the rotational speed of the stirrer were changed whereas the initial sulfide and catalyst concentration remained constant.
(Figure 1)
Kinetic curves of Na2S oxidation at different rotational speeds of the stirrer in the presence of a stilbenequinone-based catalyst are shown in Fig 1. The rate of Na2S oxidation is technically influenced when the rotation frequencies of the stirrer varies from 500 to 1200 rpm, indicating that the catalytic reaction is diffusion-controlled. On the other hand, the reaction rate did not change when the stirrer reaches a rotational speeds of 1200 rpm or over, indicating that the catalytic oxidation reaction occurs under kinetic control. As a result of which, all subsequent experiments were carried out at a stirring speed of 1400 rpm.
The effect of the supply rate of oxidant gas (which is a mixture of oxygen and argon) as well as of oxygen concentration on the catalytic oxidation rate of Na2S is illustrated in Fig. 2. It is evident that in a region bounded by the curve y = 0.0133 and the line y = 0.0376x -1,66, the catalytic oxidation rate of sulfide becomes independent of the feed rate of oxidant gas. In other words, the process occurs under kinetical control.
(Figure 2)
The chosen ratio of kerosene fraction to Na2S solution is 1:2, which is optimal for catalytic sulfide oxidation in the presence of stilbenequione (Table 1). Apparently, this result may be explained by the fact that the maximum interphase area between aqueous phase and hydrocarbon phase was formed.
(Table 1)
In order to study the kinetic regularities as well as establish the kinetic equation for the reaction of liquid-phase sulfide oxidation catalyzed by stilbenequinone, the order of this reaction was primarily determined by a differential method with varying initial concentration of the reaction reagents.
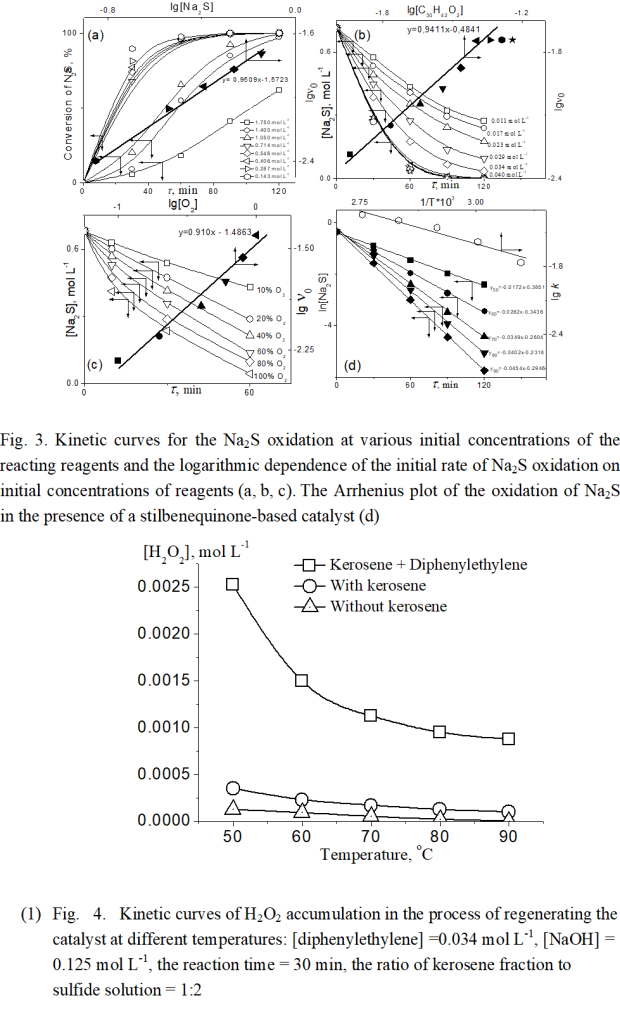
The kinetic curves of Na2S oxidation at various initial concentrations of its reactants and the plots of the logarithmic initial oxidation rate of Na2S (lgν 0) against the logarithm of reactants concentrations are shown in Figure 3 a, b, c. The initial rate of Na2S oxidation was identified from the initial slope in the reaction curve (e.i. the volume of the consumed Na2S versus reaction time).
(Figure 3)
The experiment results (Fig. 3a) show that under kinetic control the catalytic oxidation of Na2S is characterized by an induction period at initial sulfide concentration exceeding 0.7 mol L-1. In the absence of the induction period, the plot of the logarithmic initial oxidation rate of Na2S (lg0) against the logarithm of its initial concentration is a straight line with a slope of 0.9509. In other words, the reaction is first order with respect to Na2S. Similarly, the slopes of the plots of the lg0 against the logarithm of initial stilbenequinone concentration (lg [C30H42O2]) as well as of initial oxygen concentration (lg[O2]) are 0.9411 and 0.910, respectively (Fig.3b,c). This allows the conclusion that the first order of catalytic sulfide oxidation with respect to oxygen, sulfide sulfur, and stilbenequione was established. The kinetic equation of this reaction is of the following form:
ν = k [Na2S] ·[O2] ·[C30H42O2] (3)
Where ν- the catalytic oxidation rate of Na2S, k- the constant rate
As under the kinetic control the oxidation reaction of Na2S proceeds with excess of both oxygen and catalyst, the order of the reaction can be attributed to the pseudo-first one with respect to sulfide. Therefore, the overall Eq.(3) can be reduced to ν= k.[Na2S]. Based on the kinetic regularities of the first order reaction, a plot of ln [Na2S] versus reaction time t is a straight line with a slope of constant rate – k and the activation energy of the reaction was obtained from the Arrhenius lots of the rate constants k at various temperatures (50-90 oC) as given in Fig. 3d. The value of activation energy was found to be 10.11 kJ mol-1. The precoefficient in the Arrhenius equation at 90 ° C was also to be 1.29 min -1.
Mechanism of liquid-phase sulfide oxidation in the presence of a stilbenequinone-based catalyst
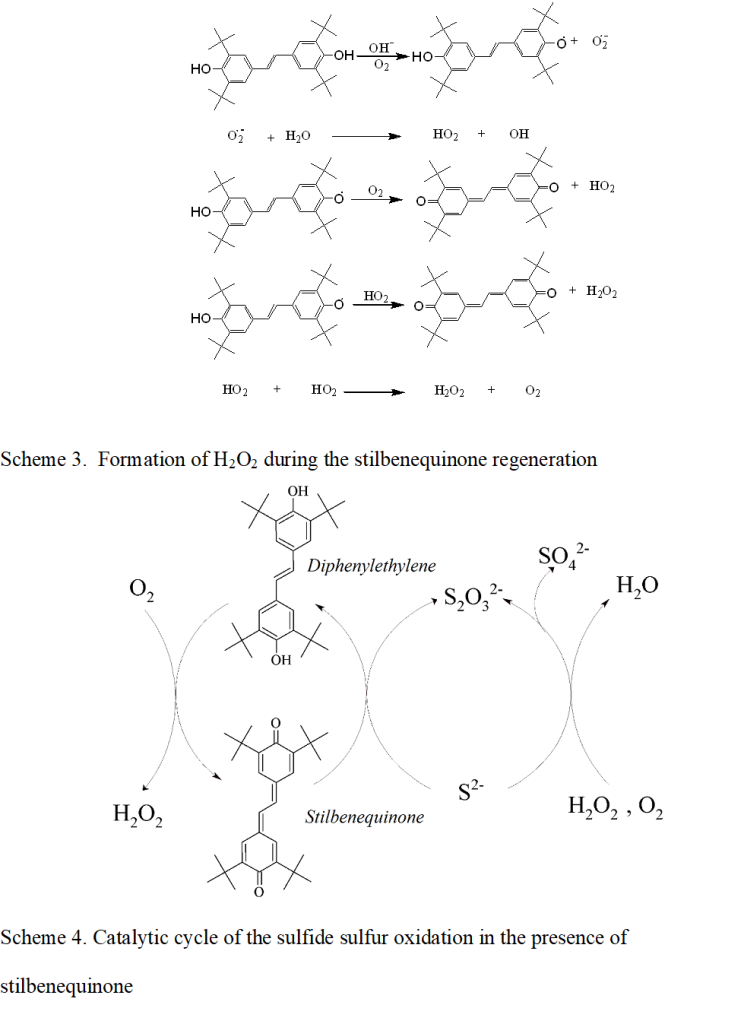
The sulfide oxidation in the presence of a catalyst based on stilbenequinone includes several stages. The research results in our previous paper [21] demonstrated that the stilbenequinone is an oxidation-reduction catalyst for the liquid-phase sulfide oxidation. The mechanism of which can proceed as follows (Scheme 2): the first stage – Na2S is hydrolyzed to NaHS ; the second stage – NaHS is oxidized by stilbenequinone to form Na2S2O3, while stilbenequinone is reduced to 3,5,3′,5′-tetra-tert-butyl-4,4′-dihydroxy-1,2-diphenylethylene (herein after referred to as diphenylethylene) ; the third stage – regeneration of catalyst by oxidizing diphenylethylene with oxygen to stilbenequinone in an alkaline medium.
(Scheme 2)
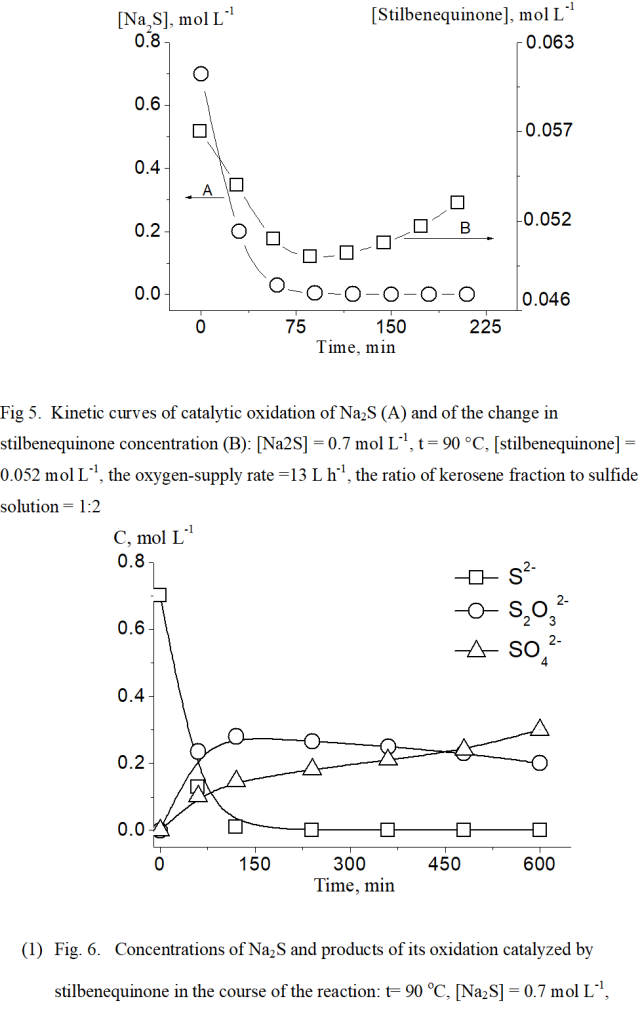
The result of analyzing intermediate and final reaction products indicated that thiosulfate and sulfate are the major products of the sulfide-oxidation reaction in the presence of stilbenequinone-based catalyst, whereas the formation of ion sulfite in this reaction was not detected. Further studies on the mechanism of catalytic sulfide oxidation, its findings revealed that a significant amount of hydrogen peroxide is formed in an aqueous-alkaline medium during the regeneration of the catalyst (Fig.4).
(Figure 4)
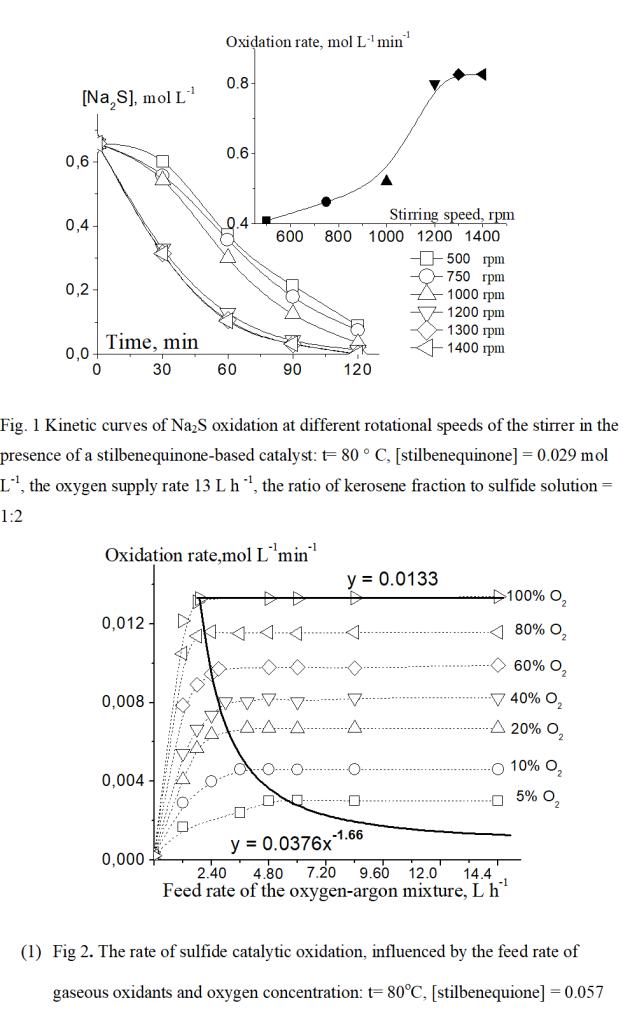
The H2O2 accumulation in the aqueous-alkaline medium has been reported in several studies [25, 26] which examined the mechanism of hindered hydroquinone oxidation in the presence of alkaline based-catalyst. The oxidative regeneration of stilbenequinone with the H2O2 formation involves several main stages, as described in Scheme 3. It is the evident about that besides hydrogen peroxide, other active forms of oxygen such as O2*-, HO2 are produced during the process of catalyst regeneration. These forms may promote oxidizing sulfide sulfur to the maximum oxidation degree (SO42-).
(Scheme 3)
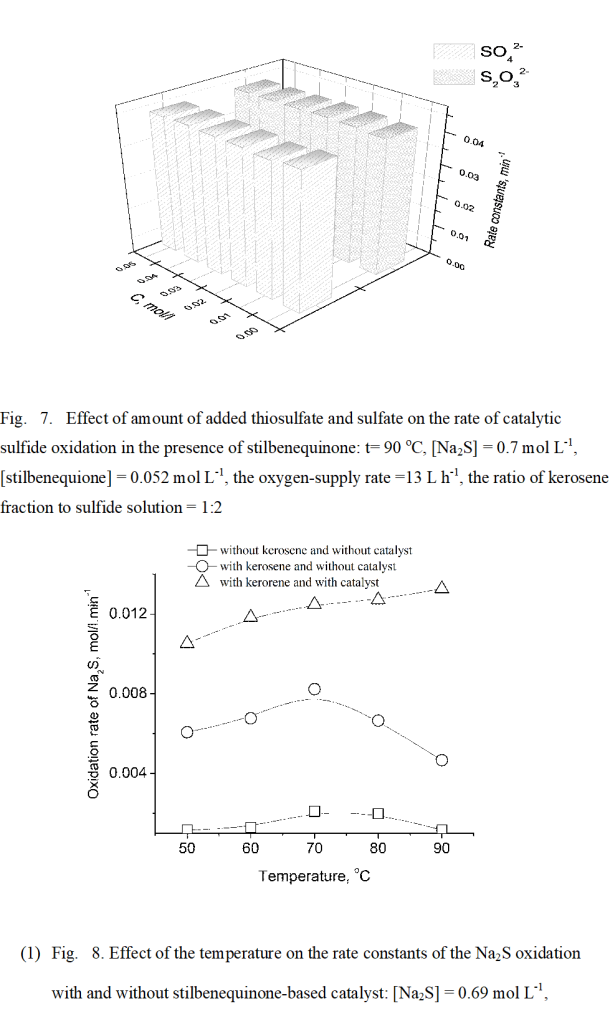
While studying the catalyst concentration change in the oxygen-saturated solution (Fig. 5), the most interesting finding was that during the reaction course the concentration of stilbenequinone decreases to a certain minimum value, and then increases solely after sulfide has reached the point of complete exhaustion. This implies that the rate of sulfide oxidation by stilbenequinone exceeds that of catalyst regeneration. In other words, the catalyts regeneration is the rate-limiting step in the oxidation reaction of sulfide sulfur in the presence of stilbenequinone.
(Figure 5)
In summary, the role of stilbenequinone in this case is to create a new and a more efficient way of tranferring electrons from the reducing agent (i.e sulfide sulfur) to the oxidizer (namely oxygen and its active forms) (scheme 4).
Factors affecting liquid-phase sulfide oxidation in the presence of a stilbenequinone based- catalyst
In general, the catalytic reaction rate depends on a number of factors: temperature, pH medium, catalyst nature and mechanism of catalyst action, etc. As the shown in Fig. 3a, when the sodium sulfide concentration is above 0.7 mol L-1, its oxidation in the presence of stilbenequinone occurs with a definite induction period. Furthermore, this period lengthens as the initial sulfide concentration increases. It is difficult to explain this phenomenon, but it might be related to the oxidation mechanism, that sulfide ion in water hydrolyzes to form hydroxide and hydrosulfide anion (1-stage), as shown in equation 4.
S2- + H2O = HS- + OH- (4)
According to Le-Chatelier’s principle,the degree of sulfide hydrolysis increases with dilution. Therefore, the dilution of Na2S concentration in the reaction solution leads to an increase in the amount of HS- ion entering into an elementary reaction with stilbenequinone (2-stage) as well as in the amount of OH- ions catalyzing the process of stilbenequinone regeneration with the formation of active oxygen forms [25, 26] (3-stage). It is a cause for increasing the number of elementary acts in the limiting stage that leads to a reduction in the induction period.
Kinetic curves of Na2S oxidation at various catalyst concentrations (Figure 3b) showed that the oxidation rate of Na2S is directly proportional to the stilbenequinone concentration in the kerosene fraction. However, this does not apply when the catalyst concentration is above 0.040 mol L-1. This result might be related to catalyst saturation in kerosene fraction. As mentioned above, the catalytic sulfide oxidation occurs at the interface between the aqueous and hydrocarbon phases (kerosene fraction). It can be seen from the data in Table 2 that the solubility of stilbenequinone in kerosene fraction only reaches 0.036 mol L-1 at 90oC. Therefore, further increasing the amount of stilbenquione in hydrocarbon phases does not influence the oxidation rate of Na2S.
(Table 2)
As discussed above, the major products of the sulfide sulfur oxidation in the presence of a catalyst based on stilbenequinone are thiosulfate and sulfate. Thiosulfate is the product of sulfide oxidation by stilbenequinone while the latter is the final product of sulfide sulfur and thiosulfate being oxidized by active oxygen forms produced during catalyst regeneration. It should be noted that the stilbenequinone does not influence the thiosulfate oxidation [21]. Moreover, one cannot exclude the possibility of partial non-catalytic oxidation of sulfide sulfur with oxygen to form sulfate in a strongly alkaline medium [15]. Obviously, as shown in Figure 6, after complete exhaustion of the sulfide in the reaction solution the thiosulfate concentration decreases meanwhile the concentration of sulfate (the final product of non-catalytic oxidation) increases. Molar mass ratio of thiosulfate to sulfate after 600 min oxidation is 3: 4.
(Figure 6)
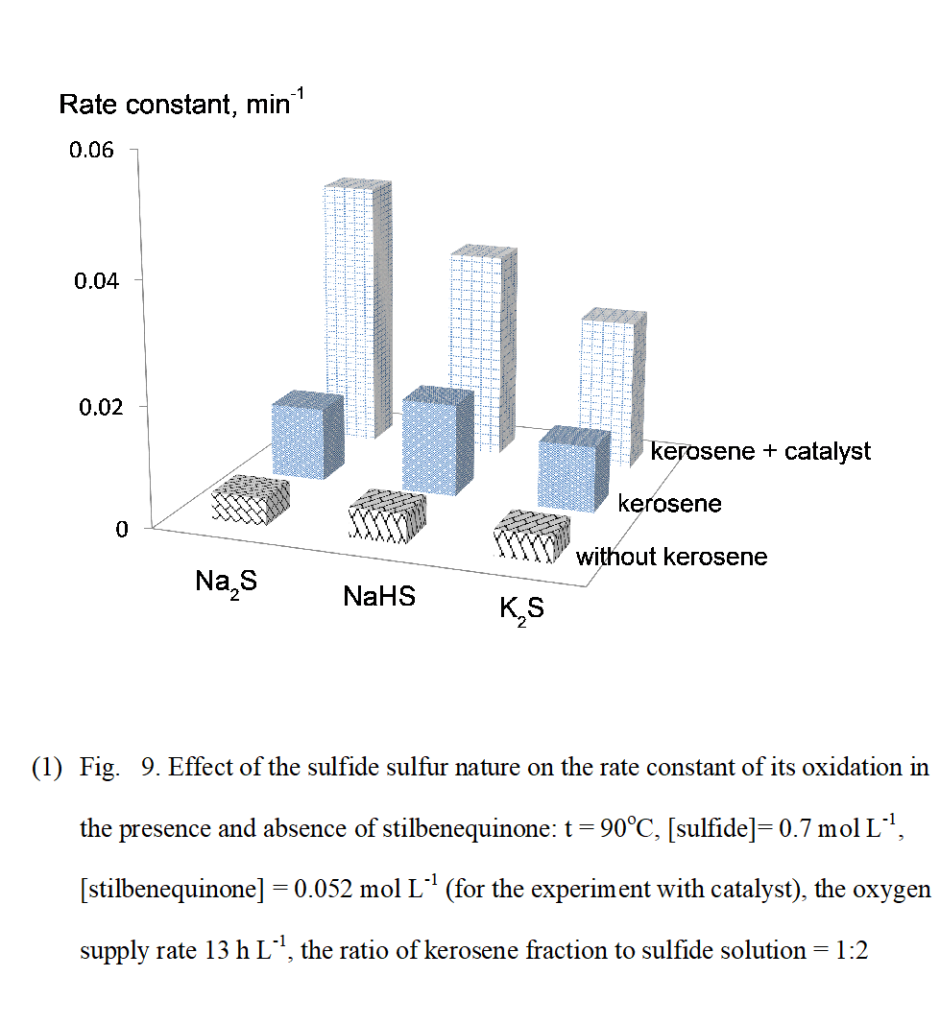
A series of experiments was performed to investigate the effect of reaction products – thiosulfate and sulfate – on the rate of catalytic sulfide oxidation. Thiosulfate and sulfate did not show any impact on the rate of sulfide oxidation reaction (Fig.7).
(Figure 7)
Oxygen simultaneously participates in two processes of the sulfide catalytic oxidation namely the catalyst regeneration and the forming of active oxygen forms. In addition, oxygen is consumed in the non-catalytic oxidation reaction of sulfur-containing substances. The effect of oxygen concentration on catalytic sulfide oxidation under diffusion control was also examined. At low oxygen concentration (i.e., 5 to 20%) the Na2S oxidation reaction catalyzed by stilbenequinone proceeded with an induction period.
Temperature was one of the most important factor affecting the rate constant of the sulfide sulfur oxidation. The internal energy of reactants increased as temperature increased and all of them then became active. In other words, the frequency of effective collisions per second between reacting molecules increases. This led to an increase in the chemical reaction rate. Figure 8 provides an overview of the effect of reaction temperature on the Na2S oxidation rate in the absence and presence of stilbenequinone based-catalyst. It is clear that in the absence of the catalyst, the sulfide oxidation rate increased as temperature grew up to 70oC and thereafter decreased when temperature grew up to 90 oC. The possible cause of this phenomenon is the decrease of dissolved oxygen in the aqueous phase as the temperature increased.
However, in the presence of the catalyst, the sulfide-oxidation rate was directly proportional to reaction temperature. A possible explanation for this might be followed: as shown in Scheme 2, the efficiency of Na2S oxidation depends on degree of its hydrolysis (1-stage) and on the rate of sulfide oxidation with stilbenequinone (2-stage) as well as on the rate of diphenylethylene oxidation with oxygen (3-stage). As the hydrolysis reaction of Na2S is endothermic, increasing the reaction temperature led to an increase of the hydrolysis degree of Na2S. Similarly, the sulfide oxidation rate with stilbenequinone [21] and the catalyst regeneration rate (presented in Table 3) also increased with increasing reaction temperature.
(Figure 8)
(Table 3)
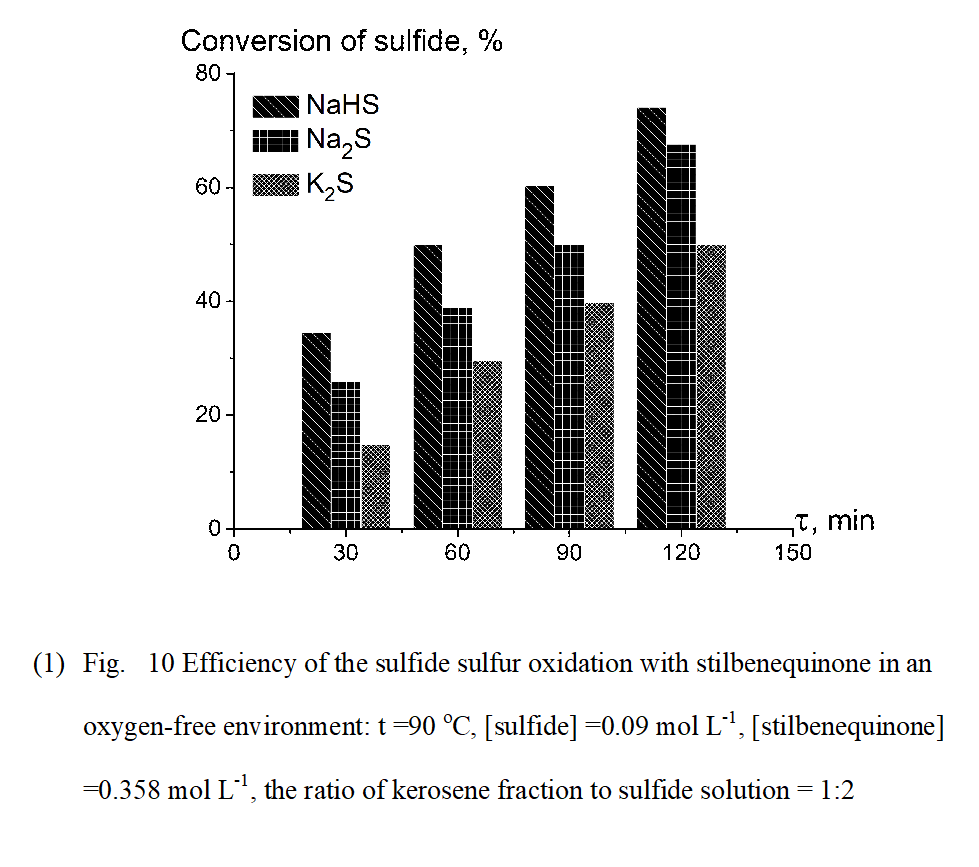
Nature of the reactants, solution pH were also important factors affecting the reaction rate. Oxidation rates of Na2S, NaHS and of K2S in the presence and absence of a catalyst based on stilbenequione were compared in Figure 9. It is known [27, 28] that a non-catalytic oxidation of sulfide sulfur with oxygen is a function of the solution pH. According to Chen and Morris [27, 28] in acid solutions (pH <6) the oxidation rate of sulfide is very slow. The specific rate increases greatly as pH increases to 8.0, and then decreases to near pH 9.0, whereupon increases again to near pH 11 and finally reduces again in more alkaline solution. From Fig.9 is evident that the rate of non-catalytic oxidation of NaHS (pH ≈9.1-9.5) is higher than that of Na2S and K2S (pH ≈13.5-14). On the other hand, in the presence of stilbenequinone the highest catalytic oxidation rate belonged to Na2S, even though the oxidation rate of NaHS by stilbenequinone in an oxygen-free environment oxidation rate exceeded that of Na2S and K2S (Fig.10). This may be due to an increase in the amount of OH- anion in Na2S and K2S solution compared to NaHS solution, which contributed to the process of catalytic regeneration with the formation of oxygen active forms.
In our opinion, the observed effect of sulfide sulfur oxidation by stilbenequinone may be related to a high concentration of cations H+ in NaHS solution (HS- + H2O = H3O+ + S2- ). It is well known that quinones are conjugated α, β-unsaturated carbonyl compounds that perform a large dipole moment with the excess negative electron density on oxygen atoms. Therefore, quinones are very sensitive to electrophiles [29]. Compared to the cations Na+ and K+, the proton H+ has a significantly higher electrophilicity index (H= 2.01 > Na =0.88 > K=0.76) [30] as well as a small radius (H+= 0.8751fm < Na+=0.09 nm < K+= 0.133nm) [31]. As a result, the proton H+ favourably attacks on the hindered carbonyl group of stilbenequinone, producing an intermediate cation that more easily forms an activated complex with the sulfide sulfur. This implies that, the rate of the sulfide sulfur oxidation with stilbenequinone in an oxygen-free environment decreases in the series: NaHS > Na2S > K2S.
(Figure 9)
(Figure 10)
Kerosene fraction in the sulfide catalytic oxidation was used as a catalyst carrier owing to its high boiling point [32] and its low solubility in water [33]. Moreover, the good solubility of stilbenequinone in this kerosene fraction was also an advantage. Consequently, the losses of this catalyst and its carrier during purification process can be minimized. Another important finding was that the presence of hydrocarbon phase led to accelerating the non-catalytic sulfide oxidation (see also Figure 8, 9). It is possible that this result is due to the higher solubility of oxygen in kerosene compared to in the aqueous medium [34]. Thus, in the catalytic sulfide oxidation the kerosene fraction played both the role of the catalyst carrier and the oxygen deposition enhancer.
Conclusion
In conclusion, the kinetics of liquid-phase oxidation of inorganic sulfides in the presence of stilbenequinone-based catalyst has been presented as a first-order reaction with respect to each reactant. The role of stilbenequinone is to create an efficient way of tranferring electrons from the sulfide to active oxygen forms produced during the process of catalyst regeneration. The result of this study indicates that the products of sulfides oxidation catalyzed by stilbenequinone such as thiosulfate and sulfate did not affect this reaction rate. Interestingly, either at low oxygen concentration or at high sulfide concentration its catalytic oxidation in the presence of stilbenequinone proceeds with an induction period. Besides, efficiency of sulfide sulfur degradation decreases in the series Na2S > NaHS > K2S.
Acknowledgements
Authors would like to express gratitude to our colleagues from R&D “AhmadullinS – Science and Technology” for providing insight and expertise that greatly assisted the research.
Reference:
1. Tchobanoglous G., Burton FL, Stensel HD. Wastewater engineering: Treatmen and reuse. New York: Met calf & Eddy; 2003.
2. Giwa SO, Ertunc S, Alpbaz M, et al. Electrocoagulation treatment of turbid petrochemical wastewater. International journal of advances in science and technology. 2012; 5(5): 23-32.
3. Artem AB, Brykin AV, Ivanov MN, et al. Анализ стратегии развитии нефтехимии до 2015 года [Analysis of the strategy for the petrochemicals development up to 2015]. Russian Chemical Journal. 2008; 52(4):4-14. Russia.
4. Fakhriev AM, Mazgaro AM, Kashevaro LA, et al. Жидкофазный процесс переработки сероводорода с получением коллоидной серы [Liquid-phase treatment of hydrogen sulfide with the formation of colloidal sulfur]. Oil refining and petrochemistry. 1986: 11; 29-31. Russia.
5. Akhmadullina AG, Kitaeva BV, Abramova NM. Локальная окислительно-каталитическая очистка сточных вод [Local oxidative catalytic wastewater treatment]. HTTM. 1988: 3; 42-44. Russia.
6. Yakovleva SV, Yakovleva YV. Водоотведение и очистка сточных вод [Wastewater and wastewater treatment]. Moscow: Stroyizdat; 2002. Russia.
7. Jan BL, Wicher TK, Willibrordus PM. Van Swaaij. The oxidation of sulfide in Aqueous Solutions. Chem. Eng. J. 1987;11: 111-120.
8. Nhi BD, Ahmadullin RM, Ahmadullina AG, et al. Investigation of factors influencing sodium sulfide oxidation in the presence of polymeric heterogeneous catalysts of transition metal oxides. Sulfur Chem. 2014; 35 (1): 74-85.
9. Bui DN, Akhmadullin RM, Akhmadullina AG, et al. Polymeric Heterogeneous Catalysts of Transition-Metal Oxides: Surface Characterization, Physicomechanical Properties, and Catalytic Activity. Chemphyschem. 2013; 14(18):4149-4157.
11. Akhmadullin RM, Bui DN, Akhmadullina AG, et al. Catalytic activity of manganese and copper oxides in the oxidation of sulfur compounds. Kinetics and Catalysis. 2013; 54(3): 334–337.
12. Zhang X, Dou G, Wang Z, et al. Selective catalytic oxidation of H2S over iron oxide supported on alumina-intercalated Laponiteclay catalysts. J. Hazard. Mater. 2013; 260;104–111.
13. Lemos BR, Teixeira IF, Machado BF, et al. Oxidized few layer graphene and graphite as metal-free catalysts for aqueous sulfide oxidation. J. Mater. Chem. A. 2013; 1: 9491–9497.
14. Wang Y, Zhao H, Li M, et al. Magnetic ordered mesoporous copper ferrite as a heterogeneous Fenton catalyst for the degradation of imidacloprid. Appl. Catal. B: Environ. 2014; 147:534–545.
15. Ramankutty CG, Sugunan S. Surface properties and catalytic activity of ferrospinels of nickel, cobalt and copper, prepared by soft chemical methods. Appl. Catal. A. 2001; 218: 39–51.
16. Cunha IT, Teixeira IF, Albuquerque AS, et al. Catalytic oxidation of aqueous sulfide in the presence of ferrites (MFe2O4, M = Fe, Cu, Co). Catal. Today.2015; 259: 222-227.
17. Kohl AL, Nielsen RB. Gas purification. Texas: Gulf Publishing Company; 1997.
18. Nicholas PC. Handbook of Air Pollution Prevention and Control. Elsevier; 2002.
19. Ueno Y, Williams A, Murry FE. A new method for sodium sulfide removal from an aqueous solution and application to industrial wastewater and sludge. Water, Air and Soil Pollution.1979; (11)1:23–42.
20. Iwasawa Y, Ogasawara S. Catalytic oxidation of hydrogen sulfide on polynaphthoquinone. J. Cat.1977.; 46 (2):132-142.
21. Hoang HY, Akhmadullin RM, Akhmadullina FY, et al. Investigation of 3,3′,5,5′-tetra-tert-butyl-4,4′-stilbenequinone-based catalyst in the reaction of liquid-phase oxidation of inorganic sulfides. Journal of Sulfur Chemistry. 2018; 39(2): 130-139.
22. James L. Manganaro. Pat. 4439411.1984.USA.
23. Lurye YY. Аналитическая химия промышленных сточных вод [Analytical chemistry of industrial wastewater] Moscow: Chemistry; 1984. Russia.
24. Popov I.P. Материаловедение [Materials Science]. Moscow: RSFSR;1955. Russia.
25. Shanina EL, Zaikov GE, Mekmeneva NA. Peculiarities of inhibiting the autooxidation of solid polypropylene with 4,4′-bis (2, 6-di-tert-butilphenol). Polym. Degrad. Stab. 1996; 51:51-68.
26. Karasch MS., Jochi BS. Reactions of hindered phenols: Baze-Catalyzed oxidations of hindered phenols. J. Org. Chem. 1957; 22(11):1439-1433.
27. Chen KY, Morris JC. Kinetics of oxidation of aqueous sulfide by oxygen Environ. Sci. Technol.1972; 6(6):529-537
28. Chen KY, Gupta SK. Formation of polysulfides in aqueous solution. Environ. Lett. 1973;4(3): 187-200.
29. Agronomists A.E. Избранные главы органической химии [Selected chapters of organic chemistry]. Moscow: Chemistry; 1990. Russia.
30. Robert GP, Szentpály LV, Liu S. Electrophilicity index. J. Am. Chem. Soc.1999; 121: 1922-1924.
31. Akhmetov N.S. Общая и неорганическая химия [General and Inorganic Chemistry]. Moscow: Higher education; 1981.Russia.
32. Shashkin P.I. Регенерация отработанных нефтяных масел [Regeneration of used mineral oils]. Moscow: Gostoptekhizdat;1960. Russia.
33. Volkov OM. Пожарная безопасность на предприятиях транспорта и хранения нефти и нефтепродуктов [Fire safety at the petroleum products enterprises]. Moscow: Nedra;1981. Russia.
34. Shaipak AA. Гидравлика и гидропневмопривод: Основы механики жидкости и газа [Hydraulics and hydropneumatic actuator: Fundamentals of fluid and gas mechanics]. Moscow: MGIU; 2003. Russia.