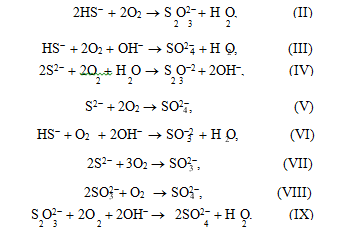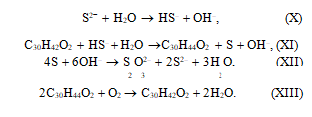Liquid-Phase Oxidation of Inorganic Sulfides in Aqueous Medium in the Presence of a Catalyst Based on 3,3′,5,5′-Tetra-tert-Butyl-4,4′-Stilbenequinone
H. Y. Hoanga, *, R. M. Akhmadullinb, F. Yu. Akhmadullinaa, R. K. Zakirova, and A. G. Akhmadullinab
Abstract—The kinetics of liquid-phase oxidation by oxygen from sodium sulfide was studied in an aqueous medium in the presence of a catalyst based on 3,3′,5,5′-tetra-tert-butyl-4,4′-stilbenequinone dissolved in ker- osene fraction. It was found that 3,3′,5,5′-tetra-tert-butyl-4,4′-stilbenequinone selectively oxidizes sodium sulfide to sodium thiosulfate. The reaction orders in sodium sulfide, oxygen, and the catalyst were deter- mined. The reaction mechanism was proposed.
Keywords: liquid-phase oxidation of sulfide, 3,3′,5,5′-tetra-tert-butyl-4,4′-stilbenequinone, reaction mecha- nism, kinetics
INTRODUCTION
Oil refineries and the enterprises of petrochemical industry are sources of a large amount of sulfur-con- taining waste, including sulfur−alkaline wastewater (SAWW). These aqueous solutions contain highly toxic concentrated sulfide compounds that must not be discharged into the environment.
To clean SAWW from sulfide sulfur in industry, liq- uid-phase catalytic oxidation by air is used. Various heterogeneous and homogeneous catalysts based on oxides, hydroxides, spinels, transition metal phthalo- cyanines, and other catalysts [1–3] are used in SAWW treatment. Other countries, mostly the United King- dom and Japan, make use of the methods of treatment to remove sulfide sulfur SAWW that are based on ben- zoquinone, anthraquinone, or naphthoquinone. These are called quinone-based methods in Russia. The disadvantages of quinone-based methods are the low catalytic activity, substantial loss of the catalyst in the course of the process, and complications related to catalyst separation from the reaction medium when the oxidative treatment of waste is complete [4–6].
In this work, we propose to use 3,3′,5,5′-tetra-tert– butyl-4,4′-stilbenequinone (henceforth for the sake of brevity, stilbenequinone or С30Н42О2) as a catalyst dis- solved in the kerosene fraction. It shows high catalytic activity and stability in alkali media. The choice of the kerosene fraction is due to its weak solubility in water, high volatility, the ability to dissolve stilbenequinone well enough, and the ease of its separation from waste. Our goal was to study the kinetics of liquid-phase oxi- dation of sodium sulfide in the presence of stilbene- quinone as a catalyst and determine the mechanism of reaction.
EXPERIMENTAL
3,3′,5,5′-tetra-tert-butyl-4,4′-stilbenequinone was synthesized as described earlier [7]. The solution of sodium hydrosulfide was obtained by the method described in [8]. Sodium sulfide (GOST 2053-77) was dissolved in water. Oxygen (GOST 5583-78), argon (GOST 10157-79) in gas cylinders, and kerosene frac- tion (GOST 10227-2013) were used.
The catalytic oxidation of inorganic sulfides by oxygen were carried out in a three-necked glass tank reactor with a volume of 150 cm3. The reactor was filled with the solution of inorganic sulfides (40 cm3) and kerosene (20 cm3) and added the necessary amount of the catalyst. Oxygen was supplied from a gas cylinder at a space velocity of 300 h–1. The solution was stirred by a thermally controlled magnetic stirrer with 500−1400 rpm. Temperature was kept constant in the range 50−90°C.
Oxidation by stilbenequinone in the inert medium was carried out in the stainless steel autoclave (150 cm3) into which 40-cm3 solution of inorganic sul- fides, 20 cm3 of kerosene, and stilbenequinone were added. Temperature was maintained at 70−110°C and a stirring rate was 1400 rpm.
In the course of experiment, stirring was switched off at regular intervals and the samples of aqueous phase were withdrawn. The amount of sulfides was determined by potentiometric titration according to GOST 22985-90. The concentration of sodium thio- sulfate and sulfite were calculated from iodometric data according to the technique described in [9]. The concentration of sodium sulfate was determined by spectrophotometry [10]. To determine the concentra- tion of 3,3′,5,5′-tetra-tert-butyl-4,4′-stilbenequinone the sample withdrawn from the kerosene phase was subjected to hundredfold dilution with toluene and its absorbance was measured using a PE5300V spectro- photometer (Ekros, Russia) at a wavelength l of 500 nm in a 10-cm wide cell. Then, we used a calibration curve.
RESULTS AND DISCUSSION
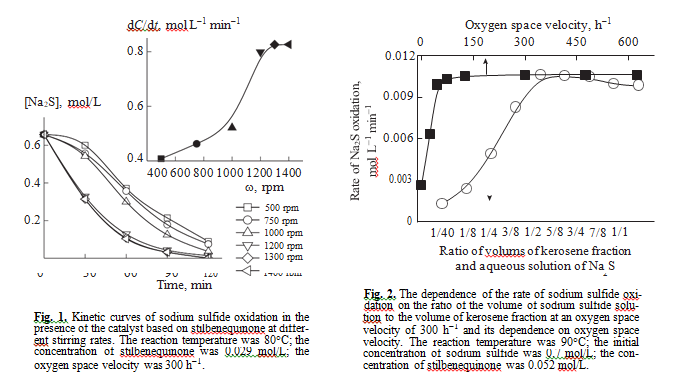
The oxidation of sulfide sulfur in the presence of the catalyst based on stilbenequinone occurs in a three-phase system oxygen–kerosene fraction–aque- ous alkali solution of sulfide sulfur. To determine where the reaction occurs, we carried out a series of experiments with varying the rate of stirring, the ratio between the volumes of kerosene fraction and the volume of the aqueous solution of sodium sulfide, and the amount of oxygen at a constant initial concentra- tion of reactants and catalysts. The inset in Fig. 1 shows that the rate of sodium sulfide oxidation depends on the intensity of stirring in the range from 500 to 1200 rpm, but above 1200 rpm, it remains con- stant. Further experiments were carried out at a stir- ring rate of 1400 rpm.
The chosen ratio of the volume of kerosene fraction to the volume of the solution of sulfide sulfur (1 : 2) in the presence of stilbenequinone is optimal. It is likely that, at this ratio, the surface of interphase interaction reaches the maximum value (Fig. 2). It was experi- mentally found that at an oxygen space velocity of
>45 h–1, oxygen does not affect the catalytic oxidation of sulfide sulfur. The results obtained allow us to con- clude that the reaction is not controlled by diffusion.
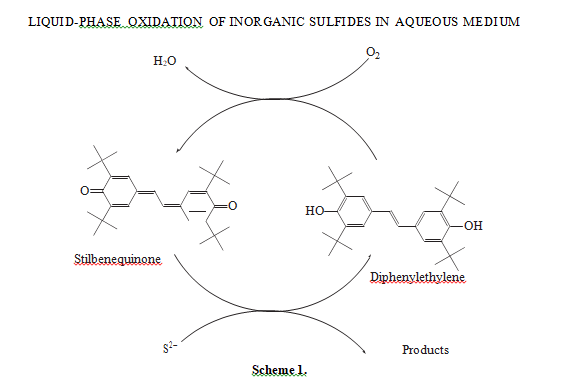
The oxidation of sodium sulfide in the presence of the catalyst based on stilbenequinone occurs via a complex mechanism. We have shown earlier [7, 11] that the catalytic cycle of sulfide sulfur oxidation in the reaction under study includes two steps: first, sulfide sulfur oxidation to thiosulfate by stilbenequinone with the reduction of the latter to 3,5,3′,5′-tetra-tert-butyl- 4,4′-dihydroxy-1,2-diphenylethylene (henceforth for the sake of brevity, diphenylethylene or С30Н44О2), and then, catalyst recovery by diphenylethylene oxidation to stilbenequinone in an alkali medium (scheme 1):
Quinones are usually strong oxidants under certain conditions [12]. In a stilbenequinone molecule, two cyclohexadiene fragments are separated by two meth- ylene groups, which stipulates the extension of a con-
Shanina et al. [15] showed that, in the liquid-phase oxidation of sodium sulfide by an organic substance, first sodium sulfide is oxidized to sulfur, which is then converted to thiosulfate via the following equation jugated system and increases electron affinity [13, 14].
6OH– + 4S ®S O2– + 2S2– + 3H O. (I)
Therefore, stilbenequinone is good two-electron oxidant capable of oxidizing sulfide sulfur with the partic- ipation of two protons. In doing so, stilbenequinone is reduced to diphenylethylene.
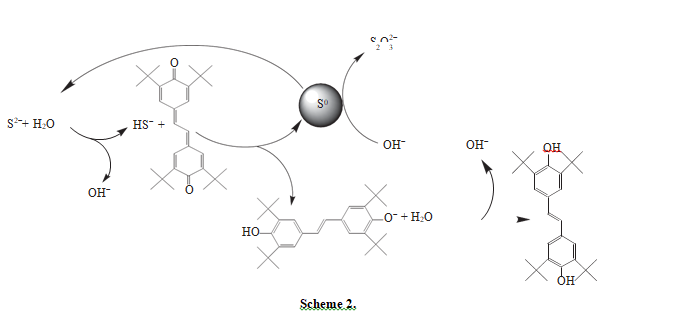
According to the proposed mechanism, the formation of thiosulfate molecule in oxidation to sulfide sulfur by stilbenequinone may occur as follows (scheme 2):
Stilbenequinone oxidizes HS− to S0 and transforms to phenoxy anion, which reacts with water and forms diphenylethylene and hydroxy anion. Then, S0 reacts with hydroxy anions and forms thiosulfate. In the course of this reaction two species of S2− are formed. Thus, to form one molecule of thiosulfate, the partic- ipation of four stilbenequinone molecules and two S2− species is needed.
Like diphenols, diphenylethylene is a hydroquinone, and thus it is sensitive to the action of oxidants. In the alkali medium that plays the role of a catalyst, hydroquinones are oxidized by oxygen to the corresponding quinones [16, 17]. The study of stilbenequi- none regeneration at different temperatures (Table 1) showed that, with an increase in temperature, the rate of diphenylethylene oxidation by oxygen increases. The role of stilbenequinone reduces to the creation of new, more efficient way of electron transfer from a reducing agent (sulfide sulfur) to the oxidant (oxygen). Kerosene in this case serves as a catalyst carrier and absorbs oxygen from the reaction medium, because the coefficient of oxygen solubility in kerosene at 20°С (0.13) is higher than in water (0.016) [18].
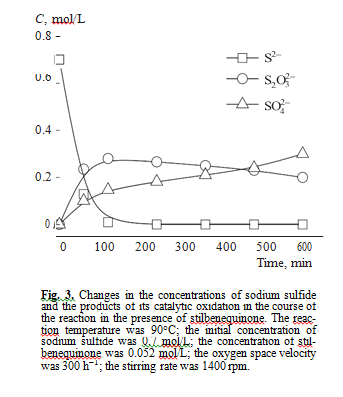
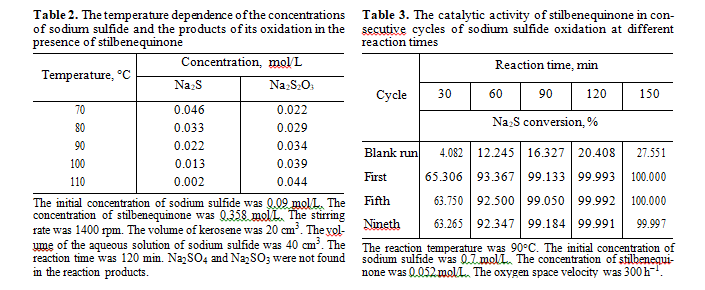
Anasysis of intermediate and final products of sul- fide sulfur oxidation in the presence of stilbenequi- none catalyst showed that the main products are sodium thiosulfate and sodium sulfate (Fig. 3). Sulfite ions were not formed.
According to literature data [19], in the catalytic oxidation of sulfide sulfur using quinones as a catalyst, noncatalytic oxidation may also occur. To check this assumption, we studied how the concentration of products of sodium sulfide oxidation change if stilben- equinone is available in the autoclave at different tem- peratures in the inert medium. As can be seen from Table 2, sodium thiosulfate is selectively formed in this reaction. Sodium sulfate and sodium sulfite were not detected. In our opinion, a sulfate ion formed in Na2S oxidation in the presence of the catalyst based on stil- benequinone is the product of noncatalytic oxidation of thiosulfate and sulfide ions by oxygen, because sul- fide ions may be oxidized to sulfate ions in strong alkali media by the following reactions [20]:
To confirm that selective synthesis of thiosulfate is possible in the catalytic oxidation of sulfide sulfur, we compared the yield of sodium sulfate formed in Na2S2O3 oxidation both in the presence and in the absence of stilbenequinone. It follows from Fig. 4 that stilbenequinone does not affect the oxidation of sodium thiosulfate.
In the aqueous medium, sulfide is hydrolyzed to hydrosulfide by reaction (X), and the latter is oxidized by stilbenequinone to sulfur by reaction (XI). Stilben- equinone is thus reduced to diphenylethylene. Sulfur is converted to thiosulfate by reaction (XII). The regeneration of stilbenequinone occurs by reaction (XIII) as a result of diphenylethylene oxidation by oxygen in an alkali medium.
Stilbenequinone has a high catalytic activity in reaction of sulfide sulfur oxidation. The rate of the cat- alytic reaction at 90°С is 17 times higher than the rate of noncatalytic oxidation. One of the important characteristics of the catalyst is its stability. The experiments confirmed that the activity of stilbenequinone remains constant after nine reaction cycles (Table 3). The turnover number, that is, the ratio of the number of moles of oxidized sulfide to the number of moles of stilbenequinone was 121.15.
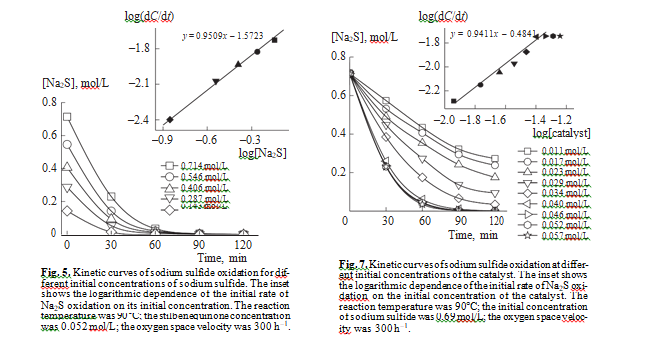
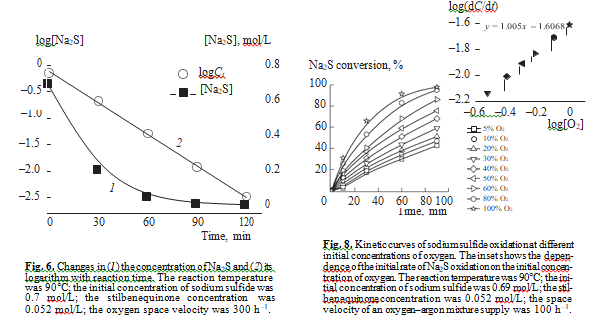
The differential method was used to determine the order of liquid phase reaction of sulfide sulfur oxida- tion in the presence of stilbenequinone at different initial concentrations of sodium sulfide. Figure 5 shows how the concentration of Na2S changes in the course of the reaction. The inset shows the logarithmic dependence of the initial rate of sodium sulfide oxidation on its initial concentration. As can be seen, this dependence is linear and the slope is 0.95. Thus, the reaction has nearly the first order in Na2S. This conclusion can be confirmed by the integral method: the linear dependence of the logarithm of Na2S concen- tration on time (Fig. 6) means that the liquidphase catalytic oxidation of sulfide sulfur in the presence of stilbenequinone is described by the first order in Na2S.
To estimate the effect of oxygen concentration in the gas/oxidant and the catalyst concentration on sul- fide sulfur oxidation, we carried out a series of experiments, in which we varied the initial concentration of stilbenequinone in the kerosene fraction and the space velocity of oxygen by diluting it with argon (Figs. 7, 8).
It follows from Fig. 7 that, with an increase in the concentration of the catalyst in the kerosene fraction from 0.011 to 0.04 mol/L, the initial rate of sodium sulfide oxidation increases. With a further increase in the amount of stilbenequinone, it remains constant. The linearity of the logarithmic dependence of the ini- tial rate of sodium sulfide oxidation from the initial concentration of the catalyst points to the firstorder reaction with respect to stilbenequinone.
Figure 8 shows how the concentration of oxygen in the gas phase affects the oxidation of sodium sulfide in the presence of stilbenequinone. When the concentration of О2 is lower than 20%, the reaction occurs with an induction period. The slope of the linear dependence of the initial rate of Na2S oxidation on log[O2] is 1.00. That is, the reaction is of the first order with respect to oxygen. As shown above, in the catalytic oxidation of sulfide sulfur, noncatalytic oxidation of sulfur-containing compounds by oxygen leads to the formation of sulfate. It has been shown in [21, 22] that the order of sulfide sulfur liquid-phase oxidation in oxygen was 0.56. The rate of sodium sulfide oxidation in the presence of stilbenequi- none is much higher than the rate of its noncatalytic oxi- dation. Therefore, we can be sure that the first order with respect to sulfide sulfur characterizes the catalytic reac- tion, and the contribution from noncatalytic oxidation is insignificant.
Thus, sulfide sulfur oxidation in the presence of a catalyst based on stilbenequinone has the first order in oxygen, stilbenequinone, and catalyst. These results confirm the proposed mechanism according to which one stilbenequinone molecule oxidizes one sulfide ion to sulfur S0 to form diphenylethylene. The latter is oxidized by one oxygen molecule in the course of catalyst regeneration. Then, S0 thus formed reacts with a hydroxide anion to form a thiosulfate molecule.
CONCLUSIONS
Thus, we found that, at a stirring rate of >1200 rpm, oxygen space velocity >45 h–1 and a ratio of the kerosene fraction to the volume of the sulfide sulfur solu- tion of 1 : 2, the catalytic oxidation of sulfide sulfur is not controlled by diffusion.
The kinetic study of sulfide sulfur oxidation in the presence of the catalyst based on stilbenequinone showed that the reaction has first order in sodium sulfide, oxygen, and a catalyst. The only product of the catalytic oxidation of sodium sulfide is sodium thio- sulfate. Sodium sulfate is formed only on the noncatalytic oxidation of Na2S.
A sequence of reactions is proposed that occur in the oxidation of sulfide sulfur in the presence of the catalyst based on stilbenequinone.
ACKNOWLEDGMENTS
The authors thank their colleagues from the research center AhmadullinS—Science and Technology for help in carrying out this study.
REFERENCES
- Akhmadullin, R.M., Bui, D.N., Akhmadullina, A.G., and Samuilov, D., Kinet. Catal., 2013, no. 3, p. 334.
- Bui, D.N., Akhmadullin, R.M., Akhmadullina, A.G., and Aghajanian, S.I., Sulfur Chem., 2013, vol. 35, p. 74.
- Bui, D.N, Akhmadullin, M., and Akhmadullina, A.G. et al., ChemPhysChem, 2013, vol. 14, no. 18, p. 4149.
- Nicholas, P C., Handbook of Air Pollution Prevention and Control, Woburn: Elsevier, 2002, p.
- Gavrilov, V., Koroleva, N.V., and Sinitsin, S.A.,
Pererabotka tverdykh prirodnykh energonositelei. Ucheb-
noe posobie (Processing of Solid Natural Energy Carri- ers. Tutorial), Digurov, N.G., Ed., Moscow: RKhTU im. D.I. Mendeleeva, 2001, p. 68.
- Kharlampovich, G.D. and Kaufmai, A.A., Tekh- nologiya koksokhimicheskogo proizvodstva. Uchebnik dlya VUZov (The Technology of Coke Production. Textbook for High Schools), Moscow: Metallurgiya, 1995.
- Hoang, Y., Akhmadullin, R.M., Akhmadullina, F.Y., Zakirov, R.K., Bui, D.N., Akhmadullina, A.G., and Gazizov, A.S., J. Sulfur Chem., 2018, vol. 39, no. 2, p. 130.
- US Patent 4439411, 1984
- Papp, J., Papperstidn., 1971, vol. 74, no. 10, p. 310.
- Lur’e, Yu., Analiticheskaya khimiya promyshlennykh stochnykh vod (Analytical Chemistry of Waste Water), Moscow: Khimiya, 1984.
- Kha, Z., Khoang, Kh.I., Akhmadulina, F.Yu., Akhmadullin, R.M., Zakirov, R.K., and Akhmadul- lina, A.G., Voda: Khim. i Ekologiya, 2017, no. 5, p. 31.
- Guben, I., Metody organicheskoi khimii (Methods of Organic Chemistry), Moscow: ONTI, 1934, vol. 3, 304.
- Bukharov, S.V., Mukmeneva, A., and Nugu- manova, G.N., Vestn. Kazanskogo Tekhnolog. Univer., vol. 5, no. 23, p. 94.
- Volod’kin, A.A. and Ershov, V., Usp. Khim., 1988, vol. 57, no. 4, p. 595.
- Jesse, B. and George, S.F., J. Am. Chem. Soc., 1933, vol. 55, no. 1, p. 232.
- Shanina, E.L. Zaikov, E., and Mekmeneva, N.A.,Polym. Degrad. Stab., 1996, vol. 51, p. 51.
- Karasch, M.S. and Jochi, B.S., Org. Chem., 1957, vol. 22, no. 11, p. 1439.
- Shaipak, A., Gidravlika i gidropnevmoprivod. Ucheb- noe posobie. Ch. 1. Osnovy mekhaniki zhidkosti i gaza (Hydraulics and Hydropneumatic Drive. Tutorial. Part
- Fundamentals of Fluid and Gas Mechanics), Mos- cow: MGIU,
- Agaev, G.A., Nasteka, I., and Seidov, Z.D., Okisli- tel’nye protsessy ochistki sernistykh prirodnykh gazov i uglevodorodnykh kondensatov (Oxidizing Processes for the Purification of Sulfur Dioxide Natural Gases and Hydrocarbon Condensates), Moscow: Nedra, 1996.
- Shtripling, L.O. and Turenko, P., Osnovy ochistki stochnykh vod i pererabotki tverdykh otkhodov. Uchebnoe posobie (Basics of Wastewater Treatment and Solid Waste Processing. Tutorial), Omsk: Izd-vo OmGTU, 2005.
- Chen, Y. and Morris, J.C., Environ. Sci. Technol., 1972, vol. 6, no. 6, p. 529.
- Jan, B.L., Wicher, K., and Willibrordus, P.M.V.S.,
Chem. Eng. J., 1987, vol. 11, p. 111.