DISTRIBUTION OF SULFUR-CONTAINING COMPOUNDS IN STREAMS FOR CONCENTRATION OF PROPYLENE
A. G. Akhmadullina, S. I. Gorodilova, A. T. Bekbulatova, V. P. Konovalov, and L. N. Orlova
UDC 665.632.074
Production of propylene is increasing steadily all over the world. For example, it increased by approximately 8 times in the USA and Japan from 1965 to 1980 [1]. Many installations for combined refining of type G-43-107 and KT-1 oil are supplemented by installations for concentration of propylene in our country for the more complete utilization of the resources of propylene contained in refinery gases. To produce propylene which satisfies the requirements of GOST 25043-81 for the concentration of sulfur compounds, it is necessary to know the group composition of these compounds and their distribution by technological streams, which will permit selecting the most economical and rational scheme for preparation of the raw material for the propylene concentration installation.
In this respect, the composition of sulfur compounds in propane-propylene fractions (PPF) processed in combined types G-43-107 and KT-1 catalytic cracking installations of the Moscow and Pavlodar Petroleum Processing Plants, and their distribution by fractions in the propylene concentration installation of the Moscow Petroleum Processing Plant, whose principal technological scheme is shown in Fig. 1, was studied. The concentration of sulfur compounds on conversion to elementary sulfur was determined with the improved VNIIUS method, which provides for a high precision of the analysis [2].
The studies showed that the concentration of sulfur compounds in PPF is directly dependent on the efficiency of hydrorefining of the raw material from the G-43-107 and KG-1 catalytic cracking installations. The concentration of thiol sulfur in PPF in normal operation of the hydrorefining section is 0.002-0.01 wt. %. The concentration of thiol sulfur in PPF increases to 0.04 wt.% in the hydrorefining section installations, and the concentration of hydrogen sulfide attains 0.01 wt.% in some periods.
The scheme for production of propylene from refinery gases includes sulfur purification and PPF rectification units, and in the case of production of a high grade of propylene, also a finished propylene drying unit. Most of the PPF is eliminated from the sulfur compounds in the sulfur purification unit with an alkaline solution, and the remainder is redistributed between the propylene and propane fractions during rectification. The data on the distribution of sulfur compounds in concentration of propylene in conditions of a poorly and satisfactorily operating hydrorefining section are reported in Table 1: the residual concentration of sulfur in the hydrorefined vacuum distillate is respectively 0.98 and 0.49 wt. %.
The analysis of the operation of the hydrorefining section overdone year at the Moscow Petroleum Processing Plant showed that the degree of sulfur purification of the vacuum distil¬late during satisfactory operation of the hydrorefining section is 60 wt.% and higher, versus 25-28 wt.% in the other cases. For a 60 wt.% degree of purification of the vacuum distillate, the concentration of thiols and carbon oxysulfides in PPF entering for concentration is respectively 16-44 and traces, 2.2 ppm, and for a 28 wt.% degree of purification, 83-350 and 6-17 ppm.
The study of the distribution of sulfur compounds between the propylene and propane fractions in the propane column (see Fig. 1) revealed a distinct feature: regardless of the initial concentration of thiol sulfur in the deethanized PPF entering for gas separation, almost 95% of the thiol sulfur is redistributed into the propane fraction (below the propane column). The concentration of thiol sulfur in the propane fraction increases by approximately three times in comparison to the concentration in PPF. Less than 5% of the thiol sulfur goes into the propylene fraction (above the propane column).
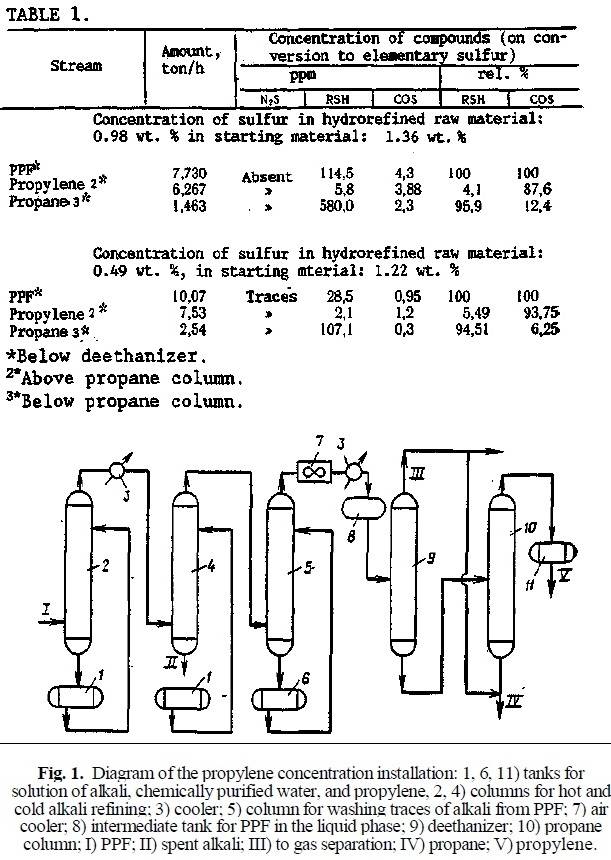
Most of the carbon oxysulfide is concentrated in the propylene (bp of 47.7°C) due to its low bp (-50°C) and only an insignificant amount (10-15% of the initial amount) is removed with the propane. The residual concentration of COS in the propane satisfies the Exxon requirements: no greater than 2 ppm [3]. In this case, the probability of total conversion of COS into H2S is very low. Propane with this concentration of COS was tested for corrosive aggressiveness (testing on a copper plate).
The data obtained on the composition of contaminants in PPF and the features of the distributions of sulfur in concentration of propylene permit selecting the most rational scheme for purification of the raw material and target fractions of the propylene concentration unit as a fraction of the requirements for the concentration of contaminant compounds. These data were used in developing the technological scheme for the propylene concentration installation at the Permnefteorgsintez Scientific Association.
LITERATURE
1. R. Chernyi, Production of Raw Material for Petrochemical Synthesis [in Russian], Khimiya, Moscow (1983), p. 21.
2. A. G. Akhmadullina et al., in: Improvement in Sulfur Purification of Hydrocarbon Raw Material and Gas Fractionati on [in Russian] f TsNIITEneftekhini, Moscow (1980), PP- 158-162.
3. M. B. Mick, Hydrocarb. Proc, 51, No. 7, 137-142 (1976).