RESEARCH IN BINARY COMPOSITIONS IN MIXTURES OF OXIDES OF TRANSITION METALS, APPLIED TO THE POLYMER MATRIX, IN THE REACTION OF THE OXIDATION OF SODIUM SULFIDE
Background
The problem of contamination of the environment is of a high importance to the whole of humanity, and due to that fact the enterprises of the fuel and energy complex are executing all measures in order to protect the environment. Ecological norms are getting more and more strict and they impose the increased requirements to all of the processes that are performed at gas and oil refining plants and enterprises which are related to the production and processing of sulfur containing substances. [1]. The sulfur-alkaline runoffs are neutralized through oxidizing the toxic sulfur containing substances and creating the less toxic products, the neutralization is performed through electro-chemical process or with the use of the chemical oxidants. [2, 3]. The most interesting method from these two is the oxidizing of the toxic sulfur containing substances with the air oxygen – this is due to its wide spread availability and low cost. The catalytic activity in this case is present in the salts of metals with variable valence, such as Ni, Mn, Cu, Co, Fe [4].
During the work we have studied oxidizing and catalytic characteristics of the oxides of the metals with variable valence applied to the polymer matrix. The use of the polymer matrix as the transport agent of the catalytic agent is related to its durability in relation to the alkalines and petrochemical products which are contained in the sulfur-alkaline runoffs. By the research that we have performed before, we have found that there is a catalytic activity of the oxides of the metals with variable valence in the reactions of oxidizing the sodium hydrosulfide. In order to increase the activity of the heterogeneous catalytic agents in the processes of the local catalytic neutralization of sulfur-alkaline runoffs it was decided to research the synergy effect of the mixed compositions of the oxides of the metals with variable valence applied to the polymer matrix, in the reaction of oxidizing sodium sulfide.
Experiments
The preparation of the catalytic agent was performed by the implementation of the oxides of the metals with variable valence into the polymer matrix in accordance with method [6]. Concentration of the oxides of the metals provided on Pic.1 was 5,0 % of the total mass. At the remaining samples, the concentration of the catalytic component in the polyethylene was in accordance with the number, marked by hyphen (Example CuO-20 – the sample of the catalytic agent with containing Cuprum Oxide – 20 % from the mass).As the polymer carrier we have used high pressure polyethylene of KAZPELEN (name of the manufacturer) make 15313-003 in accordance with GOST (State Standard) 16337-77. The heterogeneous catalytic agent was represented by cubes with the following dimensions: 2х2х2 mm.
During experiments, the following oxides of the metals with variable valence were used:
- Manganese (IV) oxide (clean) under GOST 4470-79, measure. 1-2.
- Copper (II) oxide (clean for analysis) under GOST 16539-79.
- Nickel (II) oxide (clean) under GOST 4331-78.
- Titanium (IV) oxide (clean) under GOST 9808-84.
- Vanadium (V) oxide (clean) under TC (TU) 6-09-6594-70.
- Chrome (III) oxide (clean) under GOST 2912-79.
- Molybdenum (VI) oxide (clean) under TC (TU) 2611-002-469133-2002.
- Iron (III) oxide (clean for analysis) under GOST 4173-77.
- Cobalt oxide (II, III) (clean) under GOST 4467-79.
The solutions which were tested, were prepared by the dilution of the 9-watered sodium sulfide in water under GOST 2053-77.
The oxidation of the Na2S in the alkaline solution was performed inside the chemical reactor of the bubble type. The oxygen was fed into the reaction solution with exact concentration of Na2S in presence of the catalytic agent which was being tested at a speed of 6,0 – 84,0 liter per hour.-1 The solution inside the reactor was stirred at a speed of 1400 revolutions per minute-1. The temperature of the reaction solution was maintained at 60°C with thermally controlled magnetic mixer.
The starting speed of the reaction was identified by the slope ratio of the tangent to the initial section of the curve of elimination of sodium sulfide.
The initial concentration of Na2S was 0,4 % from the mass. The concentration of the Na2S in the solution was measured by potentiometric titration under GOST 22985-90
Results and their evaluation
Influence of the nature of the oxides of the metals with variable valence on the oxidation of Na2S. The research on the effectiveness of the oxidation of the sodium sulfide in the alkaline solution at the presence of the oxides of the transition metals applied to the polymer matrix is shown on the Picture 1.
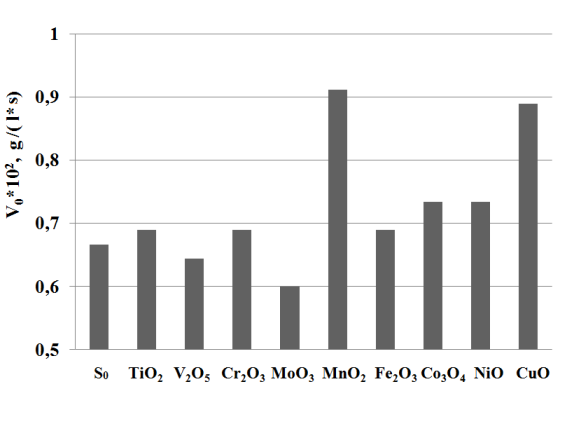
Pic. 1 Influence of the nature of the metal oxides applied to the polymer matrix at the initial speed of the oxidation (V0) of the sodium sulfide.
Maximum activity at the oxidation of the sodium sulfide is reached by the catalytic agents containing MnO2 and CuO, in the presence of which the initial speeds of the oxidation of sodium sulfide are 1,4 and 1,25 times higher (Pic.1) in comparison to the empty experiment. The catalytic agents on the basis of the metals with variable valence: Co3O4, NiO, Cr2O3, TiO2, Fe2O3demonstrate low activity, but the catalytic agents on the basis of V2O5 and MoO3 – even inhibit the oxidation of Na2S.
The results which were achieved are in compliance with the theory of two stage oxidation-reduction mechanism proposed by P.Mars and D. Kreveleny [7]. In accordance with this theory the speed of the catalytic reaction is affected by two factors:
1. The speed of the reduction of the catalytic agent depends on the energy of the chain between the Oxygen — Catalytic Agent (Cat???О):
Cat???О + R ? RO + Cat
During this process the activity of the metal oxides is placed in the following order [8]:
Co3O4>CuO>NiO>Mn2O3>Cr2O3>Fe2O3>ZnO>V2O5>TiO2
2. The speed of the formation of the catalytic agent—oxygen (Cat-O) substance, is dependent on the speed of the oxygen adsorbtion on the surface of the oxides:
2Cat + О2 ? 2Cat???О
In this case the row of activity of the oxides of the metals of the variable valence is placed in the following order [9]:
CuO>Co3O4>NiO
At the same time, the time of the most activity of the manganese oxide (IV) in the reaction of the oxidation of the sodium sulfide (pic. 1) can be explained by it’s input as an oxidant at the starting point of the reaction and as the catalytic agent during the whole time of the reaction [10]. Examination of the binary mixed compositions of the variable valency metal oxides applied to the polymer matrix, in the reaction of the oxidation of the Na2S
The research which was performed, have revealed that the activity of the variable valence metal oxides in the reaction of the oxidation of the sodium sulfide is located in the following order:
MnO2>CuO> Co3O4> NiO >Cr2O3.
In the works of the authors [11, 12] the following ability was shown: ability of the execution of the transfer of the single electron in between the cations of the metals with variable valence in the solution of a single phase oxides. Due to that fact, in order to create the more active catalytic agent we deemed necessary to study binary mixed compositions of the oxides of the metals with variable valence applied to the polymer matrix.
The results of the catalytic activity of the binary mixed compositions of the variable valence metal oxides in the reaction of oxidation of sodium sulfide are provided in the table 1. It is shown that the highest activity is received on the compositions which contain the manganese oxide (Row 1-4). At the same time, the data, which we have received from the experiments reveals the tendency of decrese in catalytic activity of the binary oxide compositions in accordance with the previously identified activity of the initial oxides. So the activity of the binary compositions is as follows: MnO2/CuO > CuO/ Co3O4 > Co3O4/ NiO > NiO/ Cr2O3.
Table. 1 Сatalytic activity of the binary mixed compositions of the variable valence metal oxides in the reaction of oxidation of Na2S.
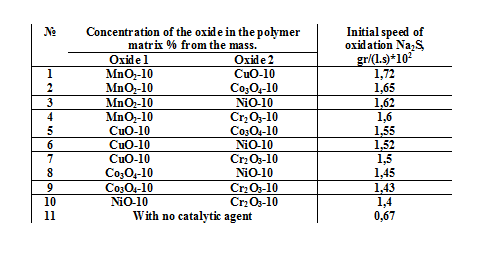
Study of the reaction of the oxidation of the Na2S in the presence of heterogeneous catalytic agent based on the oxides of copper and manganese.
Based on the maximum activity of the mixed catalytic agent – CuO-10/MnO2-10 the research was performed on the selection of optimum composition and proportions of the oxides CuO and MnO2 in the mixed composition with each other on polymer matrix. The maximum concentration of the catalytic component in the polymer matrix reached 20% of the mass. The results of the experiments are shown in picture 2.
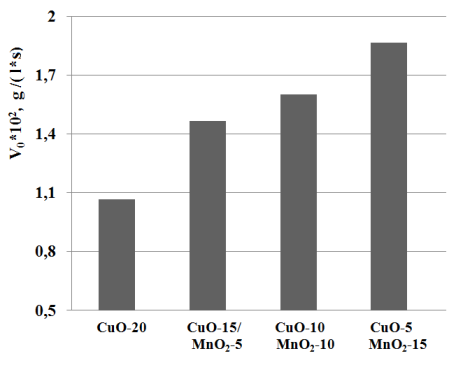
Pic. 2 The dependancy of the initial speed of oxidation (?0) from the proportion of CuO:MnO2in catalytic agent.
As it can be seen from the graphs above, it is clear that the synergy effect in the reaction of oxidation of Na2S is reached by the mixed catalytic agent of the following composition: CuO-5/MnO2-15 (1.87 g.l-1.s-1). The result that we have received is in accordance with the works of authors [11, 12] and confirms the mechanism of the transfer of the electrons between the cations of copper and manganese in the composition of the single phase oxides MnO2/CuO in accordance with the following scheme.
Mn4+ + SH? ? Mn3+ + SH° (1)
2 SH° ? HSSH (2)
Cu2+ + Mn3+ ? Cu+ + Mn4+ (3)
Cu+ + O2 ? Cu2+ + O2? (4)
Summary
1. The catalytic properties of the oxides of the metals with variable valence and their binary compositions applied to the polymer matrix in the reaction of the oxidation of sodium sulfide were identified.
2. It has been shown that the highest activity in the reaction of the oxidation of sodium sulfide is present in the binary composition on the basis of oxides of copper and manganese.
3. The mechanism of transfer of the electrons has been proposed for the transfer of electrons between the cations of copper and manganese in the compositions of the single phase oxides MnO2/CuO.
LITERATURE
[1] Abrosimov А.А. Ecology of processing of carbon hydroxide systems. М.: Chemistry. – 2002. – page 608.
[2] Akhmadullina, А.G. Neutralization and use of the sulfur-alkaline waste of oil refineries and petrochemical industry. / А.G. Akhmadullina, J.R. Abdrakhmanov, I.N. Smirnov. – Industrial overview of CNIITENeftekhim, issue 4, М. 1990, page 50.
[3] Galutkina,G.А. The use of the methods of chemical oxidation in the process of purification of the waste water of the petrochemical and oil refining enterprises. / G.А. Galutkina, А.G. Nemchenko, E.V. Rubinskaya – М.: Industrial overview of CNIITENeftekhim, 1979. –page 44.
[4] Hoffinan М. R., Lim B.C. Kinetics and mechanism of oxidation jf sulfide by oxigen: Catalitics by homogenious metalphtalocyanyne complexes // Environmental Science Technology. 1979. – v.13. – n. 11. – P. 1406-1414.
[5] Akhmadullin R.M.., Bui Din Niyi, Akhmadullina А.G., Samuilov Y.D. Catalytic activity of the oxides of the metals with variable valence applied to the polymer matrix in the reaction of oxidation of sodium bisulfate. Bulletin of Kazan technological university. – 2012. – №1.
[6] А.S. № 1041142, Bul № 34, 1983. Catalytic agent for oxidation of sulphur containing substances and methods of its preparation / А.G. Akhmadullina, А.М.Mazgarov, М.I.Alyanov, V.V.Kalacheva, I.K.Khrusheva, G.M.Nurgalieva, G.А.Ostroumova, А.F.Vildanov.
[7] Krylov, О.V. Heterogeneous catalytic reactions: Study guide for Tertiary institutions / О.V. Krylov – М.: Published by «Akademkniga», 2004. – 679 с.
[8] Boreskov, G. К. Report of Academy of Science of USSR. G.К. Boreskov, V.I. Marshneva – 1973. – Т. 213, № 1. – С. 112—115.
[9] Halpern В., Germain J. E. // Compt. rend. – 1973. – T. 277, N 24. – P. 1287 – 1290.
[10] Valeika, V. Oxidation of sulphides in tannery wastewater by use of manganese (IV) oxide / V. Valeika // Polish J. of Environ. Stud. Vol. 15 No 4. 2006, 623-629.
[11] S. Veprek, D.L. Cocke, S. Kehl and H.R. Oswald, J. Catal. 100(1986)250.
[12] F.C. Buciuman, F. Patcas, T. Hahn. A spillover approach to oxidation catalysis over copper and manganese mixed oxides. Chem. Eng. Proc. 38 (1999) 563-569.