DETOXIFICATION OF SOUR CAUSTIC WASTES ON HETEROGENEOUS PHTHALOCYANINE CATALYST
A. G. Akhmadullina, A. M. Mazgarov, I. K. Khrushcheva and G. M. Nurgalieva
UDC 628.543.49.094.3
In combatting the formation of toxic sour caustic wastes (SCW) in refineries, radical measures may be taken – a reduction of the caustic consumption in product treating 11, 2], or the complete elimination of caustic treating [3]. For a number of reasons, however, it is impossible to eliminate completely the formation of SCW. Therefore, it becomes very im¬portant to develop and implement effective methods for the detoxification and utilization of SCW.
From the standpoint of environmental protection and better utilization of the valuable chemical reagents contained in the SCW (caustic and sodium sulfide), it is of great interest to utilize the SCW in the pulp and paper industry [4]. However, the utilization of SCW formed in the treatment of LPG produced in petroleum refining and in the treatment of gasolines to remove sulfur compounds is hindered by the presence of sodium mercaptides, which have a very unpleasant odor.
Thus, in order to utilize mercaptan-containing SCW, it is necessary to develop efficient methods of deorderization. Refinery methods for detoxification of SCW may employ purging with steam or air to remove hydrogen sulfide, after preacidification of the waste stream with a mineral acid to pH 5-6 (neutralization method) [5], or they may employ treatment of the waste streams with carbon dioxide (carbonation method) [6]. The principal shortcoming of these methods is the formation of gas with a low concentration of hydrogen sulfide, which cannot be used in Claus units or in sulfuric acid production. Therefore, the waste acid gases from the waste detoxification units are burned, but this pollutes the environment with sulfur dioxide discharges. Moreover, the neutralization and carbonation methods require con¬siderable consumption of chemical reagents (acids or carbon dioxide); and the carbonation method also consumes large amounts of heat, without ensuring that the sodium mercaptides will be thoroughly removed from the waste.
Of the methods of SCW detoxification, the most interesting is the oxidation of the toxic sulfur compounds in the waste by the use of atmospheric oxygen. In the absence of catalysts, this process is performed at temperatures of 90-110°C and pressures of 0.3-0.5 MPa. The use of catalysts can give a significant acceleration of the oxidation-process, so that it can be performed at 40-50°C.
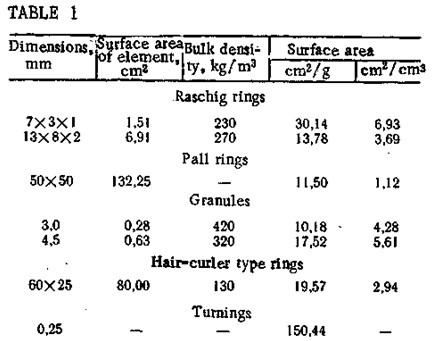
At VNIIUS, in cooperation with the Ivanovo Chemical Technology Institute, a heterogen¬eous phthalocyanine catalyst KS-1 has been developed for sulfur removal; this catalyst has a polymer base (polyethylene or polypropylene) [7J. The catalyst has a high level of chemical stability, mechanical strength, and stable catalytic activity. It is prepared in the form of granules that are then used to manufacture packing elements with a highly developed geometric surface, for example, Raschig rings, Pall rings, or “hair-curler” type rings (Table 1).
A process was developed for the heterogeneous catalytic oxidative detoxification of SCW on the KS-1 catalyst. This process consists of oxidation of sodium sulfide by atmospheric oxygen to the less harmful sodium thiosulfate and sulfate, and the oxidation of sodium mer¬captides to organic disulfides that are insoluble in the SCW and are separated from the waste stream by settling in oil traps.
The reactions of sodium sulfide and mercaptide oxidation in the kinetic region are first-order with respect to sodium sulfide and mercaptide, oxygen, and catalyst.
On the basis of the results obtained in kinetic studies, the following process condi¬tions were selected: temperature 40-50°C, pressure 0.3-0.5 MPa. The catalyst is charged to the reactor in the form of packing elements in a continuous bed with a height of 6D to 8D (where D is the reactor diameter).
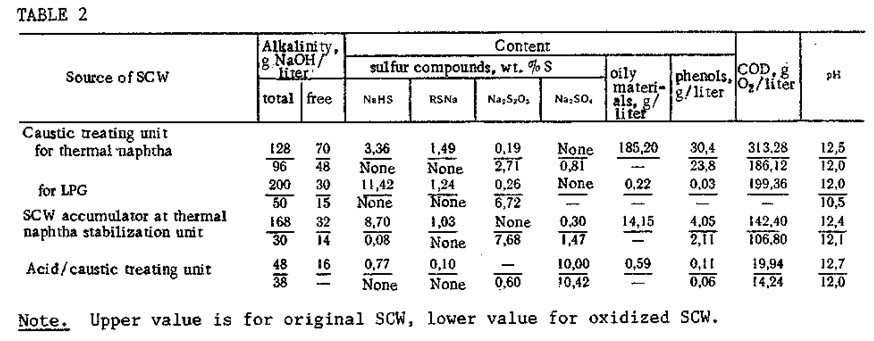
The process of heterogeneous catalytic oxidation has been used under these conditions, with a linear air feed velocity of 0.03-0.04 m/sec, to detoxify actual wastes from the Nbvo-kuibyshevsk Petrochemical Combine and the Taupse Petroleum Refinery. In Table 2 we show the change in physicochemical properties of the SCW from different process units of the Taupse refinery in the process of heterogeneous catalytic oxidative detoxification in a batch labor¬atory unit with a 0.5-liter reactor. It can be seen that the SCW oxidation is accompanied by a considerable reduction of the total and free alkalinity and the chemical oxygen demand (relative reductions of 40-50%). The oxidation lowers significantly the concentration of sodium phenolates; this is apparently related to partial oxidation of phenols and also to purging of the phenols from the system by air at a velocity of 0.04 m/sec. In the absorber solution (10% NaOH) through which the spent air was passed, there was no sodium sulfide or mercaptide, but phenolates were found in approximately 10% of their original concentration in the SCW. In the course of SCW detoxification, approximately 80% of the sodium sulfide is oxidized to sodium thiosulfate, and 20% to sodium sulfate. The oxidized wastes, in compari¬son with the original materials, do not have any unpleasant odor of hydrogen sulfide or mer-captans.
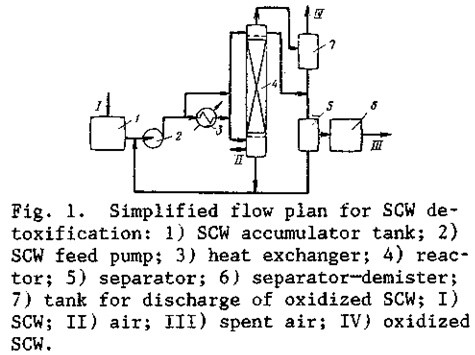
On the basis of the process test results in detoxification of actual wastes, together with a kinetic study of model reactions of the oxidation of sulfur compounds, a process flow plan has been developed (Fig. 1), along with engineering standards for the design of experim-mental-commercial units.
The SCW, separated from oily materials and solid contaminants, is fed from an equalizing tank to a heat exchanger, where the stream is steam-heated to 40°C; from there, it passes into a reactor with the KS-1 catalyst. Compressed air at a pressure of 0.3-0.5 MPa is fed into the bottom of the reactor through a sparger.
The reactor piping is arranged so that it can be operated in either cocurrent or counter-current flow. In the cocurrent scheme, partially oxidized SCW from the upper part of the reactor passes into the separator 5 for removal of entrained air from the SCW. The streams in the treating systems circulate from the separator 5 to the pump to the heat exchanger (or bypassing the heat exchanger) to the reactor and then back to the separator 5 in order to en¬sure the required degree of sodium mercaptide and sulfide removal. Then, the SCW from the separator 5 is discharged to the detoxified waste tank. In the countercurrent scheme, the SCW is fed to the top of the reactor, above the catalyst bed. In this case the reactor may operate either in the washing mode or in the bubbling mode.
The spent air, containing vapors of hydrocarbons, phenols, and disulfides entrained from the oxidized wastes, passes from the top of the reactor to the separator—demister 6, where it is separated from the entrained drops of SCW and then directed to afterburning in the near¬est process furnace. The drops of SCW entrained from the separator 6, as they accumulate, are discharged to the separator 5. The oxidized waste streams are directed through the tank 7 for mixing with other waste streams from the No. II sewer system. The organic disulfides that were formed by oxidation of mercaptides are separated from the waste streams of the No. II sewer systems, together with the oily materials, by settling in oil traps.
In the heterogeneous catalytic detoxification of SCW, there is not atmospheric pollu¬tion by any discharges of hydrogen sulfide or sulfur dioxide. The proposed method consumes little heat and does not consume any reagents (such as carbon dioxide or sulfuric acid). The method gives a more nearly complete removal of the toxic, foul-smelling sulfur compounds from the waste. The alkaline character of the reaction medium and the relatively low process tem¬perature make it feasible to use carbon steel vessels.
The anticipated economic advantage from the introduction of the liquid-phase oxidation process for detoxification of sour caustic wastes in place of the carbonation process [8], in a unit with a capacity of 10,000 metric tons per year, is 68,000 rubles per year.
LITERATURE
1. A. M. Fakhriev, L. A. Kashevarov, A. F. Vil’danov, et al., in: Summaries of Papers from 3rd Ail-Union Seminar “Improvement of Processes of Gas Fractionation and Sulfur Removal from Hydrocarbon Feedstocks,” [in Russian], TsNIITEneftekhim, Moscow (1983).
2. A. M. Mazgarov, A. V. Neyaglov, S. Sh. Telyakov, et al., Khim. Tekhnol. Topi. Masel, No. 12, 6-8 (1976).
3. E. G. Ioakimis and I. Z. Nurmukhametova, Khim. Tekhnol. Topi. Masel, No. 6, 30-33 (1981).
4. L. N. Piterskii et al., Tr. Vses. Nauchno-Issled. Inst. Org. Sint., p. 104 (1978).
5. USSR Inventor’s Certificate 524,775.
6. M. G. Rudin, G. A. Arsen’ev, and A. V. Vasil’ev, The General Plant Facilities of a Petrol¬eum Refinery [in Russian], Leningrad (1978).
7. USSR Inventor’s Certificate 1,041,142.
8. K. G. Asvlova, V. I. Nazarov, T. A. Kolesnik, et al., Khim. Tekhnol. Topi. Masel, No. 5, 12-13 (1983).